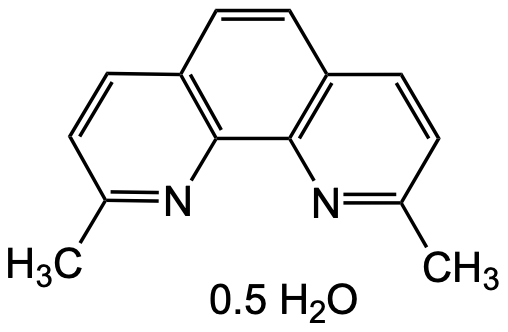
Neocuproine hemihydrate
Product Code:
CDX-N0115
CDX-N0115
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short term: +20°C, Long term: +20°C
Short term: +20°C, Long term: +20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-N0115-G025 | 25 g | £138.00 |
Quantity:
| CDX-N0115-G100 | 100 g | £393.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
2,9-Dimethyl-1,10-phenanthroline; DMPHEN; NSC 4280; VUF 7738
Appearance:
White to light yellow powder.
CAS:
484-11-7
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS05, GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H302 - H315 - H318 - H335
InChi:
InChI=1S/2C14H12N2.H2O/c2*1-9-3-5-11-7-8-12-6-4-10(2)16-14(12)13(11)15-9;/h2*3-8H,1-2H3;1H2
InChiKey:
IEBXFSLFDFHSRD-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 484-11-7. Formula: C14H12N2 . 0.5H2O. MW: 217.27. Neocuproine is a complexing reagent, known for its ability to form stable complexes with metal ions, particularly CU2+ ions. This property makes it valuable in various analytical and research applications, including spectrophotometry and electrochemistry. In the presence of CU2+ ions, neocuproine forms a brightly colored complex that can be used for the quantitative determination of copper in solution. The Neocuproine-CU2+ complex exists in a 2:1 ratio with a maximum absorption at 454 nm. Neocuproine is used in complex with Cu2+ as chromogenic oxidant in the total antioxidant capacity assay (CUPRAC method). In addition to its analytical uses, neocuproine and its derivatives have been employed in the field of coordination chemistry to study and synthesize metal complexes. These complexes often have unique properties and are of interest for their potential applications in catalysis, material science, and other areas of chemical research. This compound is also widely used in some biomedical fields, such as in the study of copper metabolism disorders and neurodegenerative diseases and neocuproine-CU2+ complexes have shown biological properties, such as antitumor activity.
MDL:
MFCD00004973
Molecular Formula:
C14H12N2 . 0.5H2O
Molecular Weight:
217.27
Package Type:
Vial
Precautions:
P280 - P301 + P312 + P330 - P302 + P352 - P305 + P351 + P338 + P310
Product Description:
Neocuproine is a complexing reagent, known for its ability to form stable complexes with metal ions, particularly CU2+ ions. This property makes it valuable in various analytical and research applications, including spectrophotometry and electrochemistry. In the presence of CU2+ ions, neocuproine forms a brightly colored complex that can be used for the quantitative determination of copper in solution. The Neocuproine-CU2+ complex exists in a 2:1 ratio with a maximum absorption at 454 nm. Neocuproine is used in complex with Cu2+ as chromogenic oxidant in the total antioxidant capacity assay (CUPRAC method). In addition to its analytical uses, neocuproine and its derivatives have been employed in the field of coordination chemistry to study and synthesize metal complexes. These complexes often have unique properties and are of interest for their potential applications in catalysis, material science, and other areas of chemical research. This compound is also widely used in some biomedical fields, such as in the study of copper metabolism disorders and neurodegenerative diseases and neocuproine-CU2+ complexes have shown biological properties, such as antitumor activity.
Purity:
>99% (Assay)
Signal Word:
Danger
SMILES:
CC1=NC2=C(C=C1)C=CC3=C2N=C(C=C3)C.CC1=NC2=C(C=C1)C=CC3=C2N=C(C=C3)C.O
Solubility Chemicals:
Soluble in methanol (50mg/ml), ethanol (10mg/ml) or DMSO (25mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
Documents
References
(1) A. Mohindru, et al.; Biochem. Pharmacol. 32, 3627 (1983) | (2) H.H. Al-Sa?doni, et al.; Br. J. Pharmacol. 121, 1047 (1997) | (3) J.G. De Man, et al.; Eur. J. Pharmacol. 381, 151 (1999) | (4) R. Apak, et al.; Free Radic. Res. 39, 949 (2005) | (5) A.A. Gouda & A.S. Amin; Arabian J. Chem. 3, 159 (2010) | (6) O.V. Patel, et al.; Biometals 26, 415 (2013) | (7) K.A. Jesse, et al.; Inorg. Chem. 58, 9057 (2019)