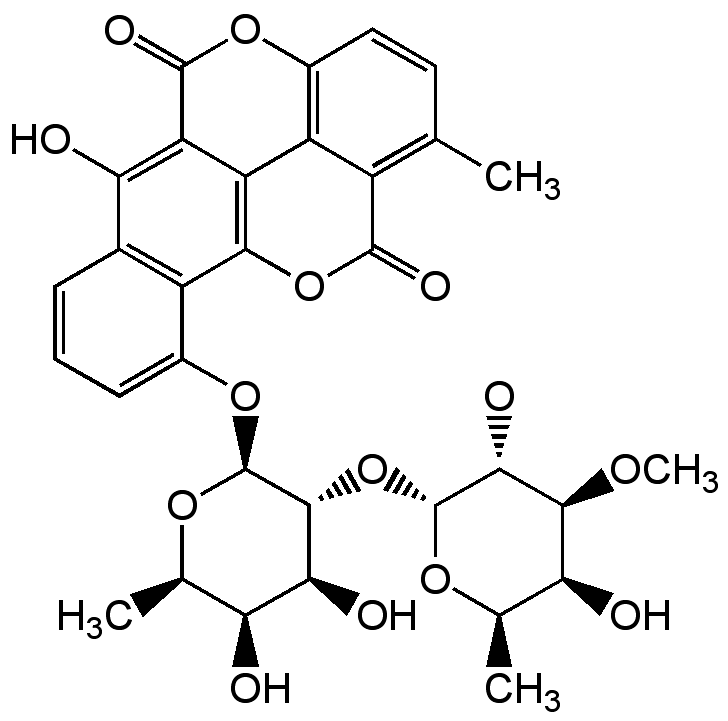
Chartreusin
Product Code:
BVT-0005
BVT-0005
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
4°C
4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0005-M005 | 5 mg | £150.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
Lambdamycin
Appearance:
Yellow solid.
CAS:
6377-18-0
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07,GHS08
Hazards:
H302, H312, H319, H351
InChi:
InChI=1S/C32H32O14/c1-10-8-9-15-18-16(10)29(38)45-26-17-13(23(35)20(19(18)26)30(39)43-15)6-5-7-14(17)44-32-28(24(36)21(33)11(2)42-32)46-31-25(37)27(40-4)22(34)12(3)41-31/h5-9,11-12,21-22,24-25,27-28,31-37H,1-4H3/t11-,12-,21+,22+,24+,25-,27?,28-,31-,32+/m1/s1
InChiKey:
PONPPNYZKHNPKZ-CIFTUGPDSA-N
Long Description:
Chemical. CAS: 6377-18-0. Formula: C32H32O14. MW: 640.6. Isolated from Streptomyces chartreusis. Antibiotic Antitumor compound. Topoisomerase II inhibitor. Induces single-strand scission in DNA in the presence of reducing agents. Apoptosis inducer. Inhibitor of glioblastoma multiforme (GBM).
MDL:
MFCD00467138
Molecular Formula:
C32H32O14
Molecular Weight:
640.6
Package Type:
Plastic Vial
Precautions:
P201, P270, P281, P301, P312, P302, P352, P405
Product Description:
Antibiotic Antitumor compound. Topoisomerase II inhibitor. Induces single-strand scission in DNA in the presence of reducing agents. Apoptosis inducer. Inhibitor of glioblastoma multiforme (GBM).
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
CO[C@H]1[C@@H](O)[C@@H](C)O[C@H](O[C@@H]2[C@@H](O)[C@@H](O)[C@@H](C)O[C@H]2OC2=CC=CC3=C2C2=C4C(C(=O)OC5=C4C(=C(C)C=C5)C(=O)O2)=C3O)[C@@H]1O
Solubility Chemicals:
Soluble in acetone; insoluble in water.
Source / Host:
Isolated from Streptomyces chartreusis.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C. After reconstitution protect from light at -20°C.
Documents
References
Chartreusin, a new antibiotic produced by Streptomyces chartreusis, a new species: B. E. Leach, et al.; J. Am. Chem. Soc. 75, 4011 (1953) | Chartreusin, a glycosidic antitumour antibiotic from Streptomyces: J.A. Beisler; Prog. Med. Chem. 19, 247 (1982) (Review) | Biochemical characterisation of elsamicin and other coumarin-related antitumour agents as potent inhibitors of human topoisomerase II: A. Lorico & B.H. Long; Eur. J. Cancer 14, 1985 (1993) | Chartreusin, elsamicin A and related anti-cancer antibiotics: J. Portugal; Curr. Med. Chem. Anticancer Agents 3, 411 (2003) (Review) | Biosynthesis of the antitumor agent chartreusin involves the oxidative rearrangement of an anthracyclic polyketide: Z. Xu, et al.; Chem. Biol. 12, 579 (2005) | In silico studies on marine actinomycetes as potential inhibitors for glioblastoma multiforme: P. Kirubakaran, et al.; Bioinformation 6, 100 (2011) | Synthetic Remodeling of the chartreusin pathway to tune antiproliferative and antibacterial activities: N. Ueberschaar, et al.; JACS 135, 17408 (2013) | Rational design of an apoptosis-inducing photoreactive DNA intercalator: N. Ueberschaar, et al.; Angew. Chem. Int. Edit. 52, 6185 (2013)