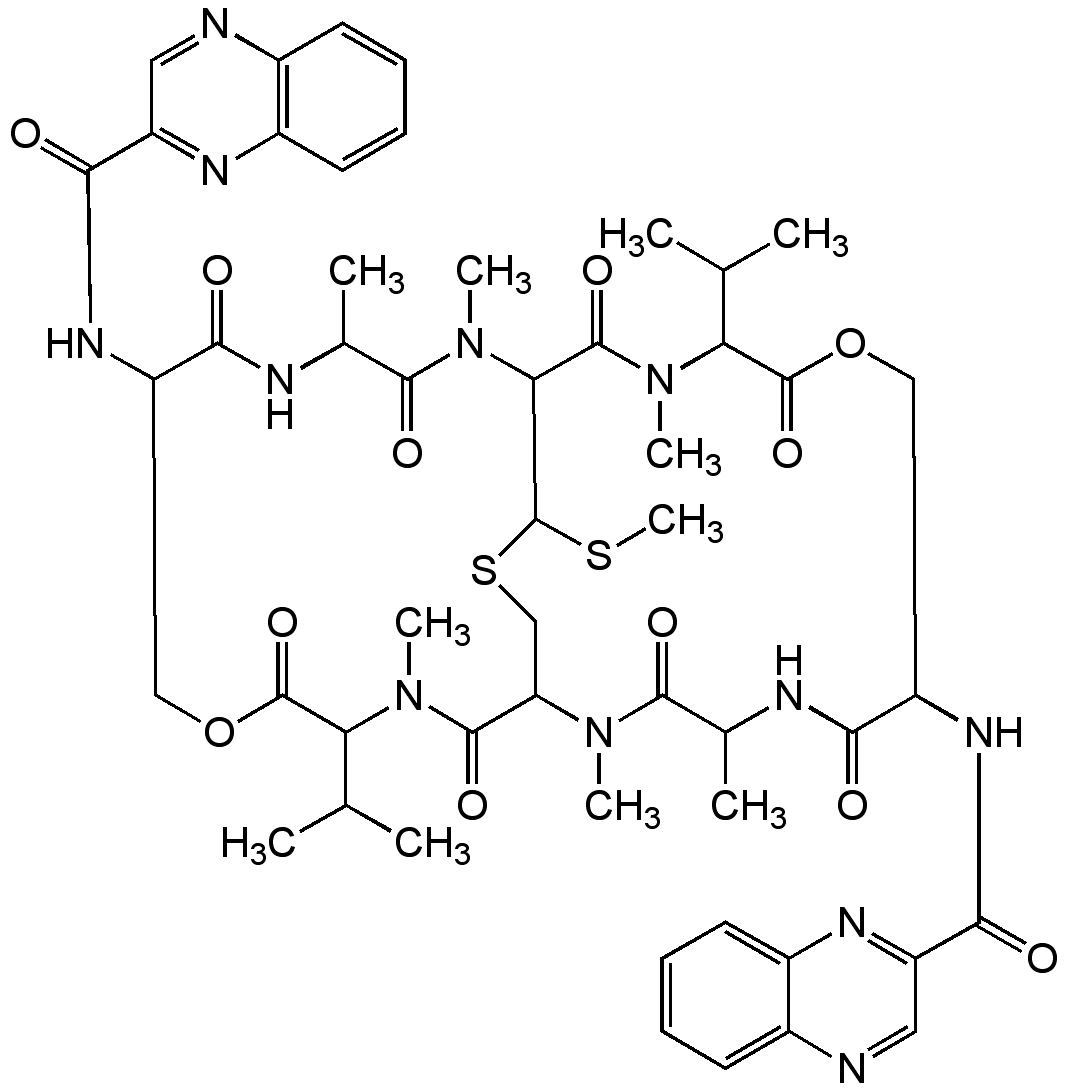
Echinomycin
Product Code:
BVT-0267
BVT-0267
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0267-M001 | 1 mg | £105.00 |
Quantity:
| BVT-0267-M005 | 5 mg | £350.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
Quinomycin A
Appearance:
White to off-white solid.
CAS:
512-64-1
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Hygroscopic.
Hazards:
H301, H311, H319, H331, H361
InChi:
InChI=1S/C51H64N12O12S2/c1-25(2)38-49(72)74-22-36(59-42(65)34-21-53-30-17-13-15-19-32(30)57-34)44(67)55-28(6)46(69)63(10)40-48(71)62(9)39(26(3)4)50(73)75-23-35(58-41(64)33-20-52-29-16-12-14-18-31(29)56-33)43(66)54-27(5)45(68)60(7)37(47(70)61(38)8)24-77-51(40)76-11/h12-21,25-28,35-40,51H,22-24H2,1-11H3,(H,54,66)(H,55,67)(H,58,64)(H,59,65)
InChiKey:
AUJXLBOHYWTPFV-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 512-64-1. Formula: C51H64N12O12S2. MW: 1101.3. Isolated from Streptomyces echinatus (DSM 40013). Antibiotic. Antitumor compound. Powerful, selective inhibitor of nucleic acid synthesis in vitro. Potent hypoxia-inducible factor 1 (HIF-1) DNA binding activity inhibitor. Apoptosis inducer. Antibacterial, antifungal and antiviral.
MDL:
MFCD00156105
Molecular Formula:
C51H64N12O12S2
Molecular Weight:
1101.3
Other data:
Contains traces of water.
Package Type:
Plastic Vial
PG:
III
Precautions:
P201, P261, P280, P301, P310, P302, P352, P310, P405
Product Description:
Antibiotic. Antitumor compound. Powerful, selective inhibitor of nucleic acid synthesis in vitro. Potent hypoxia-inducible factor 1 (HIF-1) DNA binding activity inhibitor. Apoptosis inducer. Antibacterial, antifungal and antiviral. Shown to inhibit the type I IFN?MHC class I pathway in muscle precursor cells (myoblasts).
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
CSC1SCC2N(C)C(=O)C(C)NC(=O)C(COC(=O)C(C(C)C)N(C)C(=O)C1N(C)C(=O)C(C)NC(=O)C(COC(=O)C(C(C)C)N(C)C2=O)NC(=O)C1=NC2=C(C=CC=C2)N=C1)NC(=O)C1=NC2=CC=CC=C2N=C1
Solubility Chemicals:
Soluble in acetone, DMSO (5mg/ml), methanol, dichloromethane or ethyl acetate.
Source / Host:
Isolated from Streptomyces echinatus.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
A "quinoxaline antibiotic" similar to the triostins, q.v.: I. Kuroya, et al.; J. Antibiot. 14A, 324 (1961) | The mode of action of quinoxaline antibiotics. Interaction of quinomycin A with deoxyribonucleic acid: K. Sato, et al.; J. Antibiot. 20, 270 (1967) | Echinomycin binding sites on DNA: M.M. Van Dyke & P.B. Dervan; Science 225, 1122 (1984) | Kinetic evidence that echinomycin migrates between potential DNA binding sites: K.R. Fox & M.J. Waring; Nucl. Acids Res. 13, 595 (1985) | Effect of echinomycin on DNA methylation: R.L. Adams & A. Rinaldi; FEBS Lett. 215, 266 (1987) | Echinomycin and a novel analogue induce apoptosis of HT-29 cells via the activation of MAP kinases pathway: J.Y. Park, et al.; Pharmacol. Res. 50, 201 (2004) | Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity: D. Kong, et al.; Cancer Res. 65, 9047 (2005) | Molecular signaling cascade in DNA bisintercalator, echinomycin-induced apoptosis of HT-29 cells: evidence of the apoptotic process via activation of the cytochrome c-ERK-caspase-3 pathway: J.Y. Park, et al.; Int. J. Biochem. Cell Biol. 38, 244 (2006) | Effects of the HIF1 inhibitor, echinomycin, on growth and NOTCH signalling in leukaemia cells: S. Yonekura, et al.; Anticancer Res. 33, 3099 (2013) | Echinomycin, a potential binder of FKBP12, shows minor effect on calcineurin activity: V. Singh, et al.; J. Biomol. Screen. 19, 1275 (2014) | Effects of the hypoxia-inducible factor-1 inhibitor echinomycin on vascular endothelial growth factor production and apoptosis in human ectopic endometriotic stromal cells: T. Tsuzuki, et al.; Gynecol. Endocrinol. 32, 323 (2016) | miRNA profile of neuroprotection mechanism of echinomycin in parkinson's disease: D. Kwon & H. Liew; Mol. Cell. Toxicol. 13, 229 (2017) | Molecular and cellular toxicological profiling of DNA bis-intercalator, quinoxaline compounds: echinomycin as the versatile lead: Y.-S. Park, et al.; Mol. Cell. Toxicol. 14, 9 (2018) | Encapsulation of echinomycin in cyclodextrin inclusion complexes into liposomes: in vitro anti-proliferative and anti-invasive activity in glioblastoma: W. Alshaer, et al.; RSC Adv. 9, 30976 (2019) | Design, synthesis, and conformation-activity study of unnatural bridged bicyclic depsipeptides as highly potent hypoxia inducible Factor-1 inhibitors and antitumor agents: K. Koike, et al.; J. Med. Chem. 63, 4022 (2020) | Therapeutic targeting of TP53-mutated acute myeloid leukemia by inhibiting HIF-1a with echinomycin: Y. Wang, et al.; Oncogene 39, 3015 (2020) | High-Throughput Screening to Identify Inhibitors of the Type I Interferon-Major Histocompatibility Complex Class I Pathway in Skeletal Muscle: T.B. Kinder, et al.; ACS Chem. Biol. 15, 1974 (2020) | HIF-1a is involved in blood-brain barrier dysfunction and paracellular migration of bacteria in pneumococcal meningitis: G. Devraj, et al.; Acta Neuropathol. 140, 183 (2020)