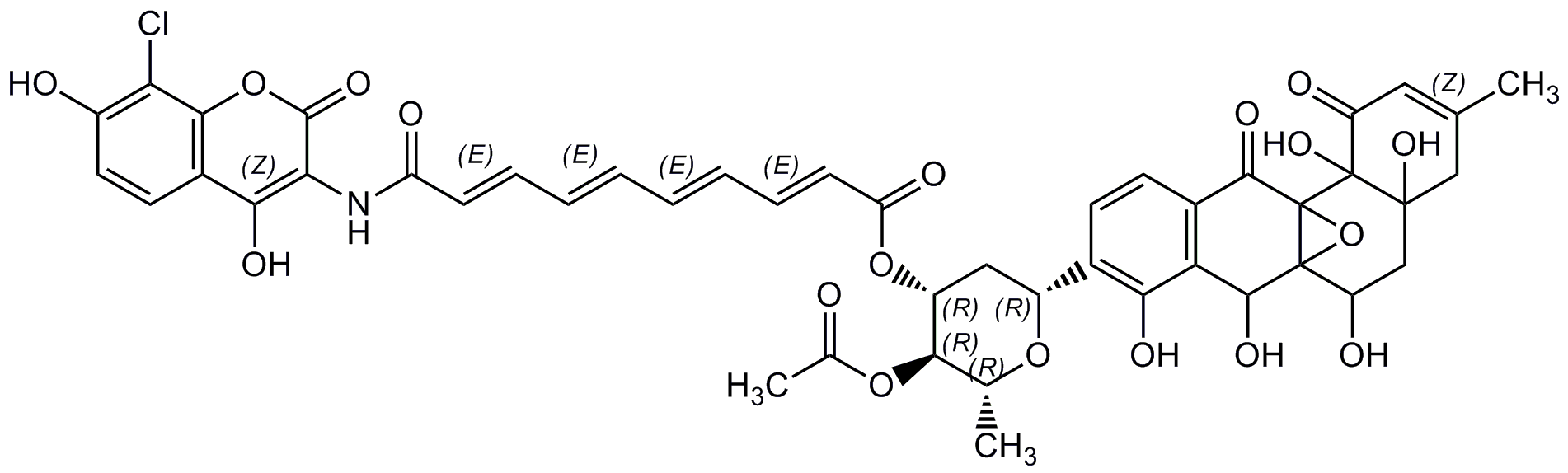
Simocyclinone D8
Product Code:
BVT-0290
BVT-0290
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0290-M001 | 1 mg | £105.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Appearance:
Yellow powder.
CAS:
301845-97-6
Class:
6.1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS06
Handling Advice:
Protect from light when in solution.
Hazards:
H300, H310, H319, H330
InChi:
InChI=1S/C46H42ClNO18/c1-20-16-29(51)45(61)43(60,18-20)19-30(52)44-41(58)33-24(40(57)46(44,45)66-44)13-12-23(36(33)55)27-17-28(38(21(2)62-27)63-22(3)49)64-32(54)11-9-7-5-4-6-8-10-31(53)48-35-37(56)25-14-15-26(50)34(47)39(25)65-42(35)59/h4-16,21,27-28,30,38,41,50,52,55-56,58,60-61H,17-19H2,1-3H3,(H,48,53)/b6-4+,7-5+,10-8+,11-9+/t21-,27-,28-,30?,38-,41?,43?,44?,45?,46?/m1/s1
InChiKey:
PLEGMCYXNQPJNV-TYXWGSLYSA-N
Long Description:
Chemical. CAS: 301845-97-6. Formula: C46H42ClNO18. MW: 932.3. Isolated from Streptomyces antibioticus T? 6040. Antibiotic. Bacterial DNA gyrase inhibitor: Staphylococcus aureus (IC50= 1.45µM), E. coli (IC50= 0.41µM). Human topoisomerase II (Topo II) inhibitor (IC50= 100µM). Antibacterial (Gram-positive). Antitumor compound.
MDL:
MFCD28899076
Molecular Formula:
C46H42ClNO18
Molecular Weight:
932.3
Package Type:
Plastic Vial
PG:
III
Precautions:
P260, P262, P280, P284, P301, P310, P302, P350, P310, P405
Product Description:
Antibiotic. Bacterial DNA gyrase inhibitor: Staphylococcus aureus (IC50= 1.45µM), E. coli (IC50= 0.41µM). Human topoisomerase II (Topo II) inhibitor (IC50= 100µM). Antibacterial (Gram-positive). Antitumor compound.
Purity:
>97% (HPLC)
Signal Word:
Danger
SMILES:
C[C@H]1O[C@H](C[C@@H](OC(=O)C=CC=CC=CC=CC(=O)NC2=C(O)C3=C(OC2=O)C(Cl)=C(O)C=C3)[C@@H]1OC(C)=O)C1=C(O)C2=C(C=C1)C(=O)C13OC1(C(O)CC1(O)CC(C)=CC(=O)C31O)C2O
Solubility Chemicals:
Soluble in DMSO, methanol, acetone, dichloromethane or ethyl acetate.
Source / Host:
Isolated from Streptomyces antibioticus T? 6040.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
Simocyclinones, novel cytostaticangucyclinone antibiotics produced by StreptomycesantibioticusTu 6040. I. Taxonomy, fermentation, isolation and biological activities: J. Schimana, et al.; J. Antibiot. 53, 779 (2000) | Biosynthesis of simocyclinone D8 in an 18O2-rich atmosphere: M. Holzenk?mpfer & A. Zeeck; J. Antibiot. 55, 341 (2002) | Simocyclinones, novel cytostaticangucyclinone antibiotics produced by StreptomycesantibioticusTu 6040 II. Structure elucidation and biosynthesis: M. Holzenk?mpfer, et al.; J. Antibiot. 55, 301 (2002) | Simocyclinone D8, an inhibitor of DNA gyrase with a novel mode of action: R.H. Flatman, et al.; Antimicrob. Agents Chemother. 49, 1093 (2005) | A crystal structure of the bifunctional antibiotic simocyclinone D8, bound to DNA gyrase: M.J. Edwards, et al.; Science 326, 1415 (2009) | In vivo and in vitro patterns of the activity of simocyclinone D8, an angucyclinone antibiotic from Streptomycesantibioticus: L.M. Oppegard, et al.; Antimicrob. Agents Chemother. 53, 2110 (2009) | Mapping simocyclinone D8 interaction with DNA gyrase: evidence for a new binding site on GyrB: C. Sissi, et al.;Antimicrob. Agents Chemother. 54, 213 (2009) | The aminocoumarins: biosynthesis and biology: L. Heide; Nat. Prod. Rep. 26, 1241 (2009) (Review) | Anti-proliferative effects of simocyclinone D8 (SD8), a novel catalytic inhibitor of topoisomerase II: A.A. Sadiq, et al.; Invest New Drugs 28, 20 (2010)