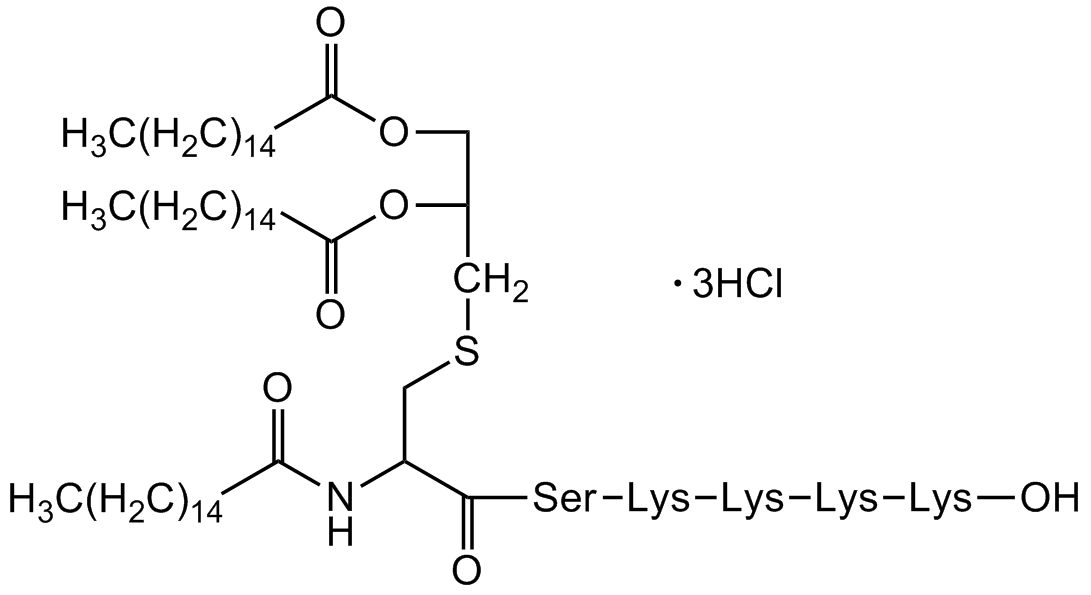
Pam3Cys-Ser-(Lys)4 . 3HCl
Product Code:
AG-CP3-0003
AG-CP3-0003
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
+4°C
+4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CP3-0003-M002 | 2 mg | £424.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges to UK mainland customers, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
Pam3Cys-Ser-(Lys)4 trihydrochloride; Pam3CSK4; Pam3Cys-SKKKK; (S)-[2,3-Bis(palmitoyloxy)-(2- RS)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH
Appearance:
Colorless powder.
CAS:
112208-04-5
Concentration:
1mg/ml after reconstitution
EClass:
32160000
Form (Short):
solid
Formulation:
Lyophilized.
GHS Symbol:
GHS07
Handling Advice:
After reconstitution, prepare aliquots and store at -20°C.Avoid freeze/thaw cycles.
Hazards:
H302, H312, H332
InChi:
InChI=1S/C81H156N10O13S/c1-4-7-10-13-16-19-22-25-28-31-34-37-40-55-73(93)86-72(65-105-64-66(104-75(95)57-42-39-36-33-30-27-24-21-18-15-12-9-6-3)63-103-74(94)56-41-38-35-32-29-26-23-20-17-14-11-8-5-2)80(100)89-71(62-92)77(97)76(96)67(51-43-47-58-82)90-91-69(53-45-49-60-84)79(99)87-68(52-44-48-59-83)78(98)88-70(81(101)102)54-46-50-61-85/h66-72,90-92H,4-65,82-85H2,1-3H3,(H,86,93)(H,87,99)(H,88,98)(H,89,100)(H,101,102)/t66-,67+,68+,69+,70+,71+,72?/m1/s1
InChiKey:
ZRKOETJPZDQIFB-OQOFFOABSA-N
Long Description:
Chemical. CAS: 112208-04-5. Formula: C81H156N10O13S . 3HCl. MW: 1510.3 . 109.4. Synthetic. Selective agonist of TLR1/TLR2 complex. Cell permeable, water soluble synthetic cationic lipohexapeptide analog of the immunologically active N-terminal portion of bacterial lipoprotein that potently activates monocytes and macrophages. Potent and effective immune adjuvant. Exerts strong local response, enhances IgG2a and IgG1 titers and upregulates proinflammatory and Th1 cytokine genes. Potent activator of the proinflammatory transcription factor NF-kappaB.
MDL:
N/A
Molecular Formula:
C81H156N10O13S . 3HCl
Molecular Weight:
1510.3 . 109.4
Package Type:
Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Selective agonist of TLR1/TLR2 complex. Cell permeable, water soluble synthetic cationic lipohexapeptide analog of the immunologically active N-terminal portion of bacterial lipoprotein that potently activates monocytes and macrophages. Potent and effective immune adjuvant. Exerts strong local response, enhances IgG2a and IgG1 titers and upregulates proinflammatory and Th1 cytokine genes. Potent activator of the proinflammatory transcription factor NF-kappaB.
Purity:
Absence of detectable protein or DNA contaminants with agonistic TLR activity.
Signal Word:
Warning
SMILES:
CCCCCCCCCCCCCCCC(=O)NC(CSCC(COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC)C(=O)N[C@@H](CO)C(=O)C(=O)[C@H](CCCCN)NN[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O
Solubility Chemicals:
Soluble in water, saline or aqueous buffers at pH <7.5.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Lipopeptide derivatives of bacterial lipoprotein constitute potent immune adjuvants combined with or covalently coupled to antigen or hapten: A. Reitermann, et al.; Biol. Chem. Hoppe Seyler 370, 343 (1989) | Induction of tumor cytotoxicity in murine bone marrow-derived macrophages by two synthetic lipopeptide analogues: P. Hoffmann, et al.; Biol. Chem. Hoppe Seyler 370, 575 (1989) | Activation of superoxide formation and lysozyme release in human neutrophils by the synthetic lipopeptide Pam3Cys-Ser-(Lys)4. Involvement of guanine-nucleotide-binding proteins and synergism with chemotactic peptides: R. Seifert, et al.; Biochem. J. 267, 795 (1990) | Synthesis of novel immunologically active tripalmitoyl-S-glycerylcysteinyl lipopeptides as useful intermediates for immunogen preparations: J. Metzger, et al.; Int. J. Pept. Protein Res. 37, 46 (1991) | The influence of various adjuvants on antibody synthesis following immunization with an hapten: J. Kellner, et al.; Biol. Chem. Hoppe Seyler 373, 51 (1992) | Lipopeptides are effective stimulators of tyrosine phosphorylation in human myeloid cells: S. Offermanns, et al.; Biochem. J. 282, 551 (1992) | Comparison of adjuvant formulations for cytotoxic T cell induction using synthetic peptides: C.E. Hioe, et al.; Vaccine 14, 412 (1996) | Cell Activation and Apoptosis by Bacterial Lipoproteins Through Toll-like Receptor-2: A.O. Aliprantis, et al.; Science 285, 736 (1999) | Adjuvant effects of various lipopeptides and interferon-gamma on the humoral immune response of chickens: M.H. Erhard, et al.; Poult. Sci. 79, 1264 (2000) | Immunostimulation by the synthetic lipopeptide P3CSK4: TLR4-independent activation of the ERK1/2 signal transduction pathway in macrophages: M.R. Muller, et al.; Immunology 103, 49 (2001) | Lipopeptide structure determines TLR2 dependent cell activation level: U. Buwitt-Beckmann, et al.; FEBS J. 272, 6354 (2005) | TLR1- and TLR6-independent recognition of bacterial lipopeptides: U. Buwitt-Beckmann, et al.; J. Biol. Chem. 281, 9049 (2006) | Human Langerhans cells selectively activated via Toll-like receptor 2 agonists acquire migratory and CD4+T cell stimulatory capacity: M. Peiser, et al.; J. Leukoc. Biol. 83, 1118 (2008)