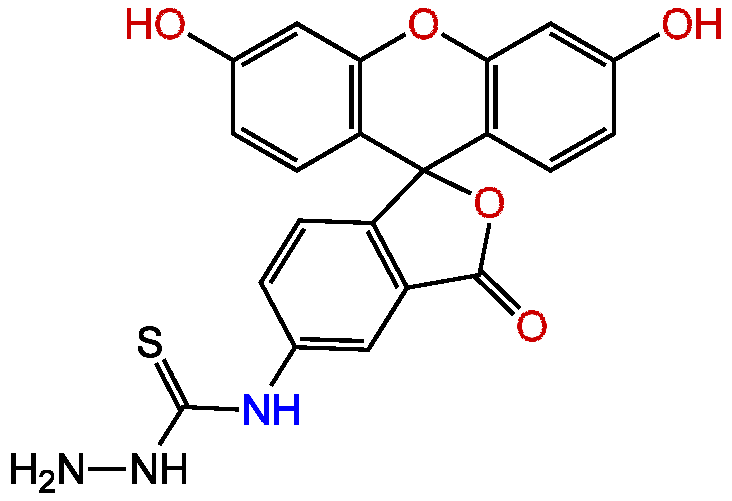
Fluorescein-5-thiosemicarbazide
Product Code:
CDX-F0005
CDX-F0005
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
-20 °C
-20 °C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-F0005-M050 | 50 mg | £126.00 |
Quantity:
| CDX-F0005-M100 | 100 mg | £218.00 |
Quantity:
| CDX-F0005-M500 | 500 mg | £863.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
5-FTSC; FTSC, FTZ; N-(3',6'-Dihydroxy-3-oxospiro[isobenzofuran-1(3H),9'-[9H]xanthen]-5-yl)-hydrazinecarbothioamide
Appearance:
Orange to brown powder.
CAS:
76863-28-0
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C21H15N3O5S/c22-24-20(30)23-10-1-4-14-13(7-10)19(27)29-21(14)15-5-2-11(25)8-17(15)28-18-9-12(26)3-6-16(18)21/h1-9,25-26H,22H2,(H2,23,24,30)
InChiKey:
OIMMDIXLTJAVTF-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 76863-28-0. Formula: C21H15N3O5S. MW: 421.43. Synthetic. Labeling of cell-surface functional groups (glycophorins). Detection of protein carbonyls in aging tissues.
MDL:
MFCD04221432
Molecular Formula:
C21H15N3O5S
Molecular Weight:
421.43
Package Type:
Vial
Product Description:
Hydrazide derivative of fluorescein. An amine-containing cell impermeant fluorescent probe that can be reversibly coupled to aldehydes and ketones on diverse molecules. This forms a Schiff base - which can be further reduced to generate stable amine derivatives by treatment with reducing agents such as sodium borohydride (NaBH4) or sodium cyanoborohydride (NaCNH3). Carboxylic acids of proteins and other water-soluble biopolymers can also be coupled to this fluorescein derivative in aqueous solution using water-soluble carbodiimides such as EDAC. Fluorescent tag for labeling of cell-surface functional groups (glycophorins) and many other diverse molecules, including DNA, RNA, polysaccharides, sialylated glycoproteins, carbonylated proteins, carbonyl derivatives, and N-acetylneuraminic acid. Useful for determining protein and peptide topology on the cell surface. This probe has been used for directly imaging of mucin-type O-linked glycoproteins within living cells, for imaging polysaccharides transported in living cells and for fluorescence labeling of short RNA by oxidation at the 3?-End. Spectral data: lambdaex=492nm; lambdaem=516nm (in 0.1 M Tris pH9.0).
Purity:
>97% (NMR)
SMILES:
NNC(=S)NC1=CC2=C(C=C1)C1(OC2=O)C2=C(OC3=C1C=CC(O)=C3)C=C(O)C=C2
Solubility Chemicals:
Soluble in water or DMF.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) D.H. Atha, et al.; Biochim. Biophys. Acta 785, 1 (1984) | (2) B. Ahn, et al.; Anal. Biochem. 161, 245 (1987) | (3) W.A. Duijndam, et al.; J. Immunol. Methods 109, 289 (1988) | (4) J.D. Corbett, et al.; Biophys. J. 66, 25 (1994) | (5) T.P. Wu, et al.; Nucleic Acids Res. 24, 3472 (1996) | (6) X. Gao, et al.; Bioorg. Chem. 25, 163 (1997) | (7) N. Tamilarasu, et al.; Bioconjug. Chem. 12, 135 (2001) | (8) A.R. Chauduri, et al.; Mech. Ageing Developm. 127, 849 (2006) | (9) Y. Zhang, et al.; Carbohydr. Res. 346, 2156 (2011) | (10) Y. Zhang, et al.; Glycoconj. J. 29, 445 (2012) | (11) Y. Zhang, et al.; Eurp. Food. Res. Technol. 239, 867 (2014) | (12) M. Have, et al.; Plant Biol. 17, 973 (2015) | (13) C. Qiu, et al.; Methods Mol. Biol. 1297, 113 (2015) | (14) R.W. Sabnis; Handbook of Fluorescent Dyes and Probes p224 (2015) | Oral absorption characteristics and mechanisms of a pectin-type polysaccharide from Smilax china L. across the intestinal epithelium: Y. Zhang, et al.; Carbohydr. Polym. 270, 118383 (2021)
Related Products
| Product Name | Product Code | Supplier | 2',7'-Difluorofluorescein | CDX-D0087 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofluorescein diacetate | CDX-D0122 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| O'-(Carboxymethyl)fluoresceinamide | CDX-D0201 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| N-(5-Fluoresceinyl)-maleinimide | CDX-F0004 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6-(Fluorescein-5(6)-carboxamido)hexanoic acid | CDX-F0015 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fluorescein diacetate | CDX-F0027 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||