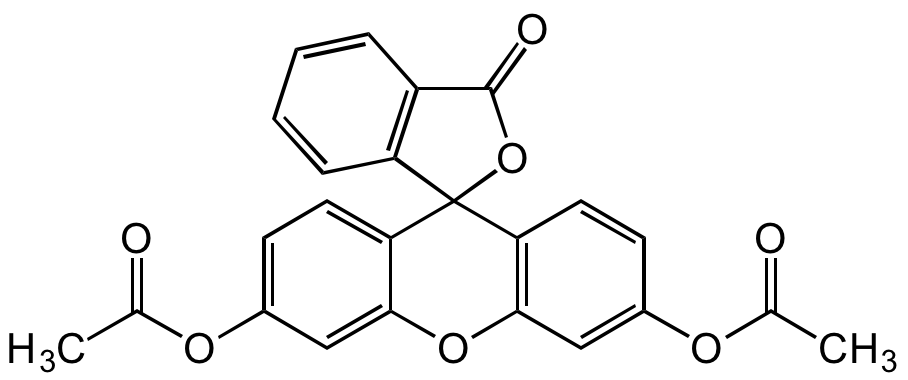
Fluorescein diacetate
Product Code:
CDX-F0027
CDX-F0027
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
-20 °C
-20 °C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-F0027-G010 | 10 g | £89.00 |
Quantity:
| CDX-F0027-G025 | 25 g | £169.00 |
Quantity:
| CDX-F0027-G100 | 100 g | £493.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
FDA; 3,6-Diacetoxyfluoran; Di-O-Acetylfluorescein; Cellstain-FDA; NSC 4726; NSC 667259
Appearance:
White to off-white powder.
CAS:
596-09-8
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C24H16O7/c1-13(25)28-15-7-9-19-21(11-15)30-22-12-16(29-14(2)26)8-10-20(22)24(19)18-6-4-3-5-17(18)23(27)31-24/h3-12H,1-2H3
InChiKey:
CHADEQDQBURGHL-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 596-09-8. Formula: C24H16O7. MW: 416.38. Synthetic. FDA is a non-fluorescent hydrophobic fluorescein derivative that can pass through the cell membrane whereupon intracellular esterases hydrolyze the diacetate group producing the highly fluorescent product fluorescein. The fluorescein molecules accumulate in cells that possess intact membranes so the green fluorescence can be used as a marker of cell viability. Cells that do not possess an intact cell membrane or an active metabolism may not accumulate the fluorescent product and therefore do not exhibit green fluorescence. FDA may be used in combination with PI staining as the non-viable cells take up the PI and stain dead cells red whereas viable cells do not take up the PI and should only stain green. This 2-color separation of non-viable and viable cells may provide a more accurate quantitation of cell viability than single color analysis. FDA is used in studies on intracellular interactions and membrane permeability, for flow cytometry and as esterase substrate. Spectral data: lambdaex=494nm, lambdaem=521nm.
MDL:
MFCD00005062
Molecular Formula:
C24H16O7
Molecular Weight:
416.38
Package Type:
Vial
Product Description:
FDA is a non-fluorescent hydrophobic fluorescein derivative that can pass through the cell membrane whereupon intracellular esterases hydrolyze the diacetate group producing the highly fluorescent product fluorescein. The fluorescein molecules accumulate in cells that possess intact membranes so the green fluorescence can be used as a marker of cell viability. Cells that do not possess an intact cell membrane or an active metabolism may not accumulate the fluorescent product and therefore do not exhibit green fluorescence. FDA may be used in combination with PI staining as the non-viable cells take up the PI and stain dead cells red whereas viable cells do not take up the PI and should only stain green. This 2-color separation of non-viable and viable cells may provide a more accurate quantitation of cell viability than single color analysis. FDA is used in studies on intracellular interactions and membrane permeability, for flow cytometry and as esterase substrate. Spectral data: lambdaex=494nm, lambdaem=521nm.
Purity:
>95% (NMR)
SMILES:
O=C(C1=C2C=CC=C1)OC32C4=C(C=C(OC(C)=O)C=C4)OC5=CC(OC(C)=O)=CC=C53
Solubility Chemicals:
Soluble in DMSO, ethanol, water, chloroform or acetone (25mg/ml).
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) B. Rotman, et al.; PNAS 55, 134 (1966) | (2) H.R. Hulett, et al.; Science 166, 747 (1969) | (3) K.H. Jones, et al.; J. Histochem. Cytochem. 33, 77 (1985) | (4) K. McGinnes, et al.; J. Immunol. Methods 86, 7 (1986) | (5) D.D. Ross, et al.; Cancer Res. 49, 3776 (1989) | (6) E. Prosperi; Histochem. J. 22, 227 (1990) | (7) M. Miyamoto, et al.; Cell Transplant 9, 681 (2000) | (8) S. Wanandy, et al.; J. Microbiol. Methods 60, 21 (2005) | (9) J. Wang, et al.; Drug Metab. Dispos. 39, 1329 (2011) | (10) N. Saruyama, et al.; Anal. Biochem. 441, 58 (2013)
Related Products
| Product Name | Product Code | Supplier | 2',7'-Difluorofluorescein | CDX-D0087 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofluorescein diacetate | CDX-D0122 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| O'-(Carboxymethyl)fluoresceinamide | CDX-D0201 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| N-(5-Fluoresceinyl)-maleinimide | CDX-F0004 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6-(Fluorescein-5(6)-carboxamido)hexanoic acid | CDX-F0015 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||