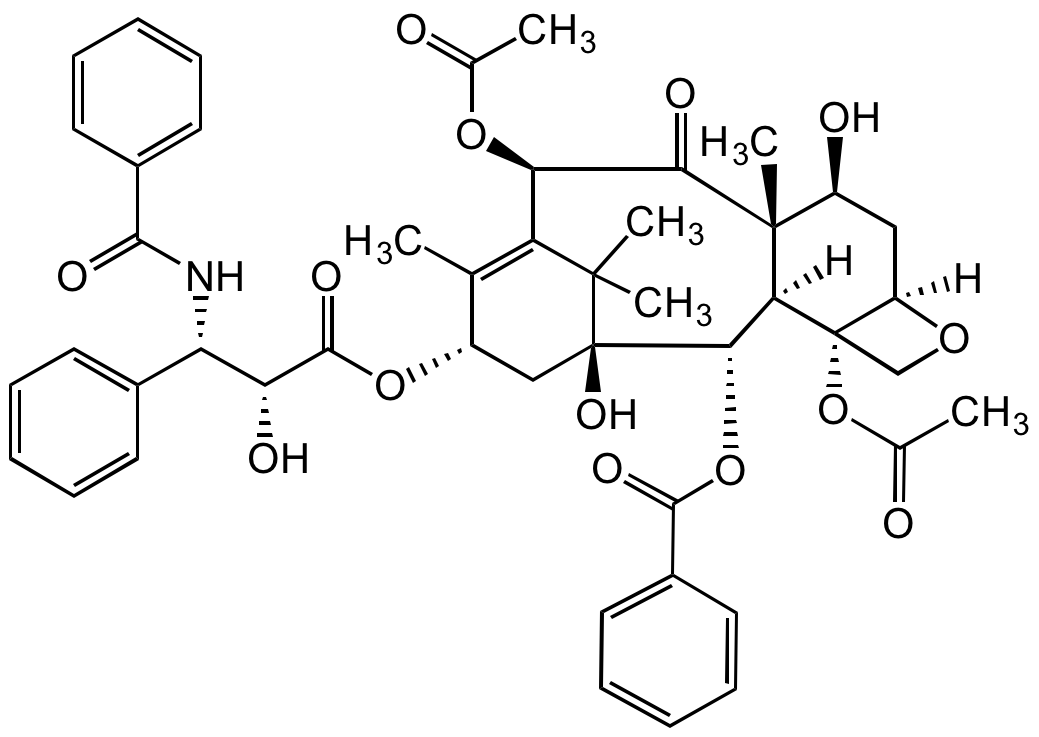
Paclitaxel
Product Code:
CDX-P0188
CDX-P0188
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
+4°C
+4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-P0188-M010 | 10 mg | £72.00 |
Quantity:
| CDX-P0188-M050 | 50 mg | £169.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Taxol; BMS 181339-01; NSC 125973
Appearance:
White to off-white powder.
CAS:
33069-62-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H341-H360-H371-H413
InChi:
InChI=1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)5/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)/t31-,32-,33+,35-,36+,37+,38-,40-,45+,46-,47+/m0/s1
InChiKey:
RCINICONZNJXQF-MZXODVADSA-N
Long Description:
Chemical. CAS: 33069-62-4. Formula: C47H51NO14. MW: 853.91. Synthetic. Anticancer compound. Chemotherapeutic used in patients with cancer and advanced forms of Kaposi's sarcoma. Microtubule assembly stabilizer. Reversibly binds to polymerized tubulin. Mitotic spindle assembly, chromosome segregation and cell division inhibitor. Induces cell cycle arrest at the G2/M phase. Apoptosis inducer through aberrant activation of cyclin-dependent kinases (CKDs) and the c-Jun N-terminal kinase/stress activated protein kinase (JNK/SAPK). Immunosuppressor, immunostimulant and TRAIL sensitizer.
MDL:
MFCD00869953
Molecular Formula:
C47H51NO14
Molecular Weight:
853.91
Package Type:
Vial
Precautions:
P201-P260-P280-P308 + P313
Product Description:
Anticancer compound. Chemotherapeutic used in patients with cancer and advanced forms of Kaposi's sarcoma. Microtubule assembly stabilizer. Reversibly binds to polymerized tubulin. Mitotic spindle assembly, chromosome segregation and cell division inhibitor. Induces cell cycle arrest at the G2/M phase. Apoptosis inducer through aberrant activation of cyclin-dependent kinases (CKDs) and the c-Jun N-terminal kinase/stress activated protein kinase (JNK/SAPK). Immunosuppressor, immunostimulant and TRAIL sensitizer.
Purity:
>98%
Signal word:
Danger
SMILES:
O[C@](C(C)(C)C1=C(C)[C@H]2OC([C@@H]([C@H](C3=CC=CC=C3)NC(C4=CC=CC=C4)=O)O)=O)(C2)[C@H]([C@]([C@]([C@@H](O)C5)(C)C([C@@H]1OC(C)=O)=O)([H])[C@]6([C@]5([H])OC6)OC(C)=O)OC(C7=CC=CC=C7)=O
Solubility Chemicals:
Soluble in DMSO (50 mg/ml), methanol (50 mg/ml) or ethanol.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
Documents
References
(1) J.E. Liebmann, et al.; Br. J. Cancer 68, 1104 (1993) | (2) R. Foa, et al.; Int. J. Clin. Lab. Res. 24, 6 (1994) (Review) | (3) M. Woods, et al.; Mol. Med. 1, 506 (1995) | (4) A. Jordan, et al.; Med. Res. Rev. 18, 259 (1998) | (5) L.A. Amos & J. L?we; Chem. Biol. 6, R65 (1999) (Review) | (6) M.V. Blagosklonny & T. Fojo; Int. J. Cancer 83, 151 (1999) | (7) T.H. Wang, et al.; J. Biol. Chem. 274, 8208 (1999) | (8) T.H. Wang, et al.; Cancer 88, 2619 (2000) | (9) T.M. Mekhail & M. Markman; Expert Opin. Pharmacother. 3, 755 (2002) | (10) A. Javeed, et al.; Eur. J. Pharm. Sci. 38, 283 (2009) (Review) | (11) D. Finlay, et al.;PLoS One 5, e13375 (2010) | (12) B. Ai, et al.; Am. J. Cancer Res. 6, 1624 (2016) (Review)
Related Products
| Product Name | Product Code | Supplier | Bromobutide | CDX-B0124 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flumetsulam | CDX-F0080 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Flupyrsulfuron-methyl sodium | CDX-F0083 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||