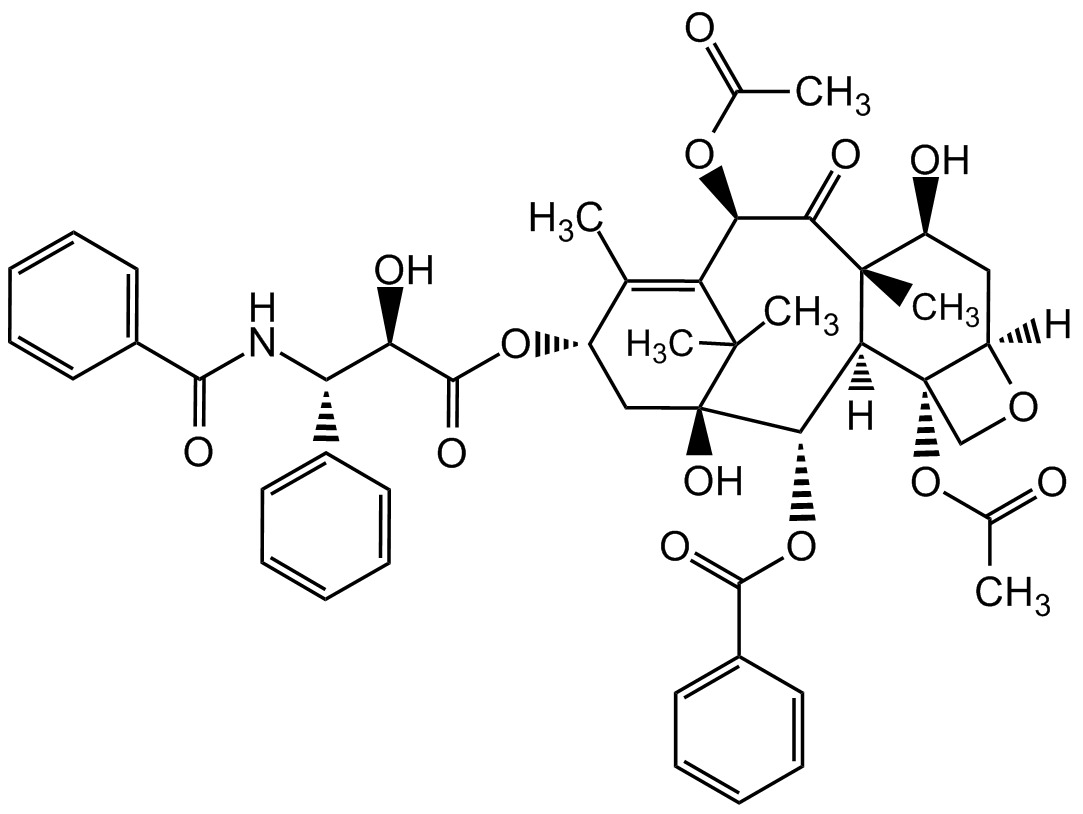
Paclitaxel
Product Code:
AG-CN2-0045
AG-CN2-0045
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
+4deg;C
+4deg;C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-0045-M001 | 1 mg | £30.00 |
Quantity:
| AG-CN2-0045-M005 | 5 mg | £40.00 |
Quantity:
| AG-CN2-0045-M025 | 25 mg | £90.00 |
Quantity:
| AG-CN2-0045-M100 | 100 mg | £165.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Taxol; BMS 181339-01; NSC 125973
Appearance:
White powder.
CAS:
33069-62-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05,GHS07,GHS08
Handling Advice:
Keep cool and dry.
Hazards:
H315, H317, H318, H335, H361, H371, H413
InChi:
InChI=1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)5/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)/t31-,32-,33+,35-,36+,37+,38-,40-,45+,46-,47+/m0/s1
InChiKey:
RCINICONZNJXQF-MZXODVADSA-N
Long Description:
Chemical. CAS: 33069-62-4. Formula: C47H51NO14. MW: 853.9. Isolated from the bark of the pacific yew tree (Taxus brevifolia). Anticancer compound. Chemotherapeutic used in patients with cancer and advanced forms of Kaposi's sarcoma. Microtubule assembly stabilizer. Reversibly binds to polymerized tubulin. Anti-mitotic. Mitotic spindle assembly, chromosome segregation and cell division inhibitor. Induces cell cycle arrest at the G2/M phase. Apoptosis inducer through aberrant activation of cyclin-dependent kinases (CKDs) and the c-Jun N-terminal kinase/stress activated protein kinase (JNK/SAPK). Immunosuppressor. Immunostimulant. Antiproliferative agent for the prevention of restenosis. TRAIL sensitizer
MDL:
MFCD00869953
Molecular Formula:
C47H51NO14
Molecular Weight:
853.9
Package Type:
Vial
Precautions:
P261, P273, P302, P352, P310
Product Description:
Anticancer compound [1, 11, 14]. Chemotherapeutic used in patients with cancer and advanced forms of Kaposi's sarcoma [11, 12]. Microtubule assembly stabilizer. Reversibly binds to polymerized tubulin [2, 3, 6, 13, 14]. Anti-mitotic. Mitotic spindle assembly, chromosome segregation and cell division inhibitor. Induces cell cycle arrest at the G2/M phase [4, 5, 10, 13]. Apoptosis inducer through aberrant activation of cyclin-dependent kinases (CKDs) and the c-Jun N-terminal kinase/stress activated protein kinase (JNK/SAPK) [7-9, 10]. Immunosuppressor. Immunostimulant [15]. Antiproliferative agent for the prevention of restenosis [16]. TRAIL sensitizer [17]
Purity:
>99% (HPLC)
Signal word:
Danger
SMILES:
[H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)C5=CC=CC=C5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@@H](NC(=O)C1=CC=CC=C1)C1=CC=CC=C1
Solubility Chemicals:
Soluble in DMSO, ethanol or acetonitrile.
Source / Host:
Isolated from the bark of the pacific yew tree (Taxus brevifolia).
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Cytotoxic studies of paclitaxel (Taxol®) in human tumour cell lines: J.E. Liebmann, et al.; Br. J. Cancer 68, 1104 (1993) | Taxol (paclitaxel): mechanisms of action: S.B. Horwitz; Ann. Oncol. 5, S3 (1994) (Review) | Taxol (paclitaxel): a novel anti-microtubule agent with remarkable anti-neoplastic activity: R. Foa, et al.; Int. J. Clin. Lab. Res. 24, 6 (1994) (Review) | Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway: C.M. Woods, et al.; Mol. Med. 1, 506 (1995) | Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle: A. Jordan, et al.; Med. Res. Rev. 18, 259 (1998) | How Taxol stabilises microtubule structure: L.A. Amos & J. L?we; Chem. Biol. 6, R65 (1999) (Review) | Mechanisms of Taxol-induced cell death are concentration dependent: K. Torres & S.B. Horwitz; Cancer Res. 58, 3620 (1998) | Molecular effects of paclitaxel: myths and reality (a critical review): M.V. Blagosklonny & T. Fojo; Int. J. Cancer 83, 151 (1999) | Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK) dependent and -independent pathways in ovarian cancer cells: T.H. Wang, et al.; J. Biol. Chem. 274, 8208 (1999) | Paclitaxel-induced cell death: where the cell cycle and apoptosis come together: T.H. Wang, et al.; Cancer 88, 2619 (2000) | Paclitaxel in cancer therapy: T.M. Mekhail & M. Markman; Expert Opin. Pharmacother. 3, 755 (2002) | Overcoming multidrug resistance in taxane chemotherapy: R. Geney, et al.; Clin. Chem. Lab. Med. 40, 918 (2002) | Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action: M. Abal, et al.; Curr. Cancer Drug Targets 300, 193 (2003) | Microtubule-stabilizing natural products as promising cancer therapeutics: B.M. Gallagher Jr.; Curr. Med. Chem. 14, 2959 (2007) | Paclitaxel and immune system: A. Javeed, et al.; Eur. J. Pharm. Sci. 38, 283 (2009) (Review) | Anti-proliferative compounds for the prevention of restenosis: M.C. Lavigne; Curr. Pharm. Des. 16, 3989 (2010) | Novel HTS strategy identifies TRAIL-sensitizing compounds acting specifically through the Caspase-8 apoptotic axis: D. Finlay, et al.;PLoS One 5, e13375 (2010)
Related Products
| Product Name | Product Code | Supplier | Latrunculin A | AG-CN2-0027 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|