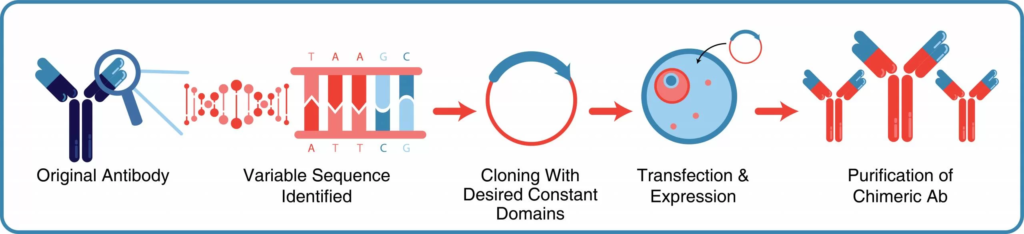
Recombinant chimeric antibodies offer many advantages over those produced from traditional hybridoma technology. These include reduced immunogenicity, the ability to mute effector functions to avoid unwanted side effects, and decreased potential for genetic drift. The combination of these factors makes recombinant antibodies an excellent choice for pre-clinical in vivo functional studies where prolonged and repeated administration of these antibodies is necessary.
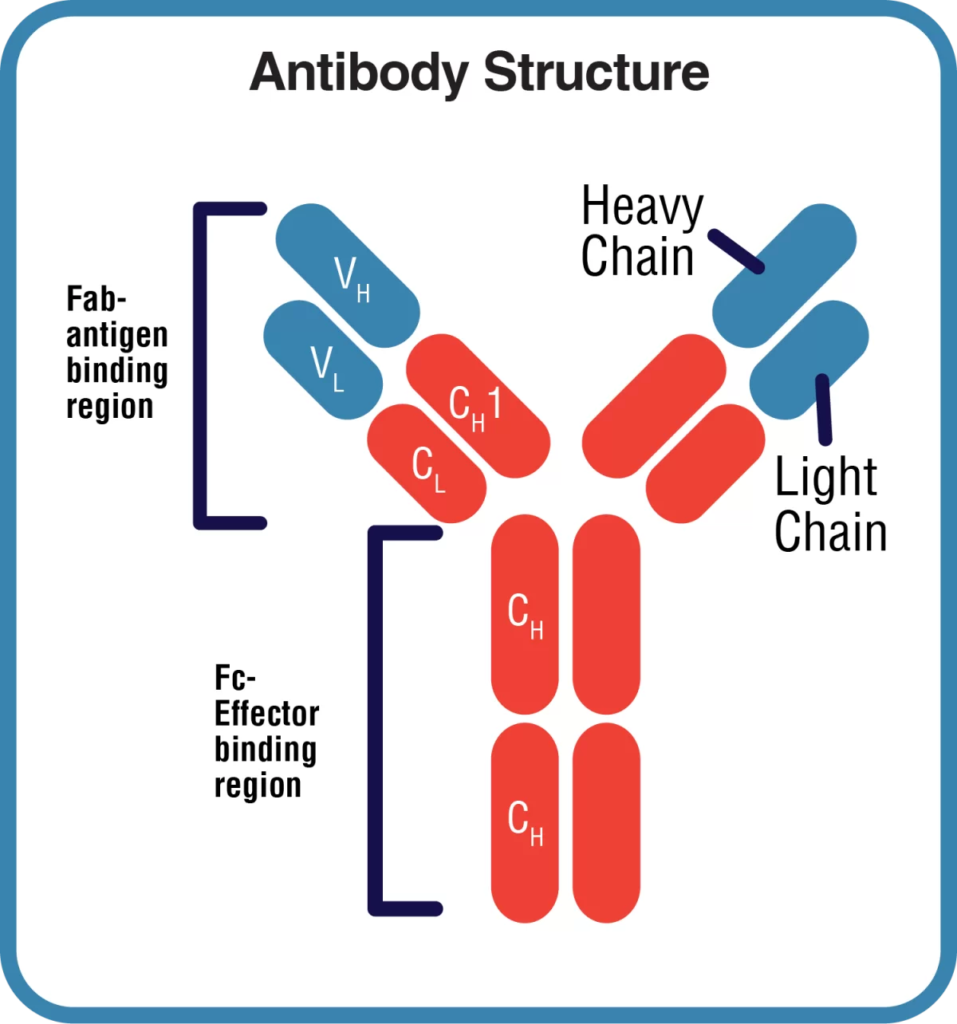
mAbMods™ recombinant antibodies have been engineered to have identical antigen binding variable domains to those of the traditional clones from which they are derived, but the IgG constant regions have been engineered to be of mouse origin. This reduces immunogenic responses in the mouse, which can lead to adverse immunological reactions and gradual loss of activity.
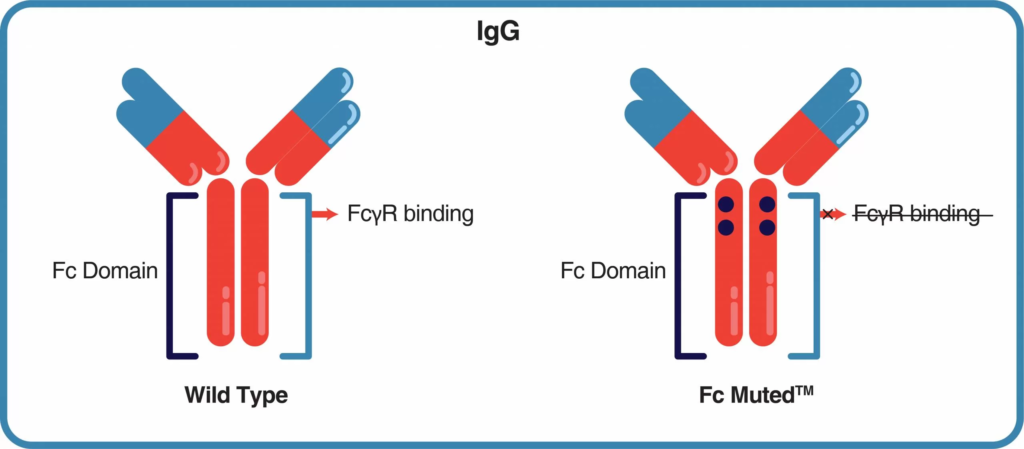
Fc Muted mAbMods™ recombinant chimeric antibodies have engineered mutations (LALA-PG or D265A) in the Fc domain that reduce or eliminate the FcγR binding. This reduces Fc-mediated effector functions such as Antibody-Dependent Cellular Cytotoxicity (ADCC), Antibody-Dependent Cellular Phagocytosis (ADCP), and Complement-Dependent Cytotoxicity that can lead to unwanted side effects.
Originally posted by Leinco Technologies Inc. on: https://www.leinco.com/chimeric-antibodies/
Caltag Medsystems is the distributor of Leinco Technologies’ products in the UK and Ireland. If you have any questions about these products, please contact us.
