The significance of antibody-based in vivo studies
For both research and therapeutic purposes, antibodies can be used to affect cellular processes in vivo and are referred to as functional/in vivo grade antibodies. There is a wide range of applications for in vivo antibody studies. Depletion antibody-based in vivo studies use surface makers like CD8 or CD4 to deplete immune cells to understand the cellular function exerted by these cell types. Other uses of in vivo grade antibodies are for activation of cellular pathways by binding to cell surface receptors or ligands such as OX-40 or Tumour Necrosis Factor (TNF). These applications are typically used for investigating signalling pathways and the outcome of downstream cellular processes such as triggering apoptosis and membrane destruction or immune cell activation. Alternatively, in vivo grade antibodies are utilised for inhibition of cellular function by blocking a receptor or ligand to stop its effect within the body as PD-1 blocks exhaustion markers in turn allows for subsequent favoured immune activation. As a result of antibody-based in vivo studies, there is a remarkable increase in the development of monoclonal antibodies to treat several diseases like multiple sclerosis, rheumatoid arthritis, and different cancers. Consequently, there is a need to highlight antibody-based studies and stress the importance of appropriate controls, especially in vivo studies.
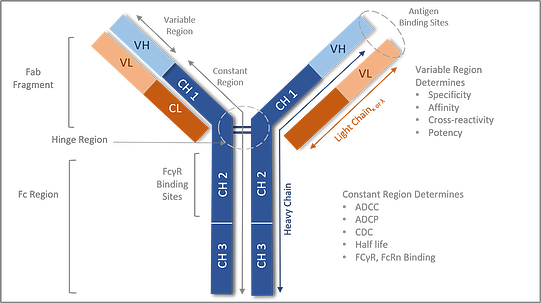
Figure 1: Components of the Antibody structure
The antibody structure consists of two heavy chains and two light chains joined by a di sulfide hinge region. The antibody can be separated by Fab arm and Fc region. The Fab arm has the antigen binding site whereas the Fc region determines the effector function activity.
What is an antibody and how does it function?
All antibody molecules share the same basic structural characteristics but display remarkable variability in the regions that bind antigens. Looking at the antibody structure (Fig.1): the antibody molecule is composed of heavy and light chains made up of the fab and Fc regions. The Fab fragment is made from heavy chains and light chains. The variable region of the heavy and light chains determines the antigen recognition, hence one of the major functions of the Fab region as well as specificity, affinity, and potency.
The Fc region is the C-terminal region of the heavy chain of an antibody that mediates the binding of the immunoglobulin to host tissues or factors, including binding to Fc receptors located on various cells of the immune system (e.g., effector cells) or to the first component (Clq) of the classical complement system. The Fc- region mediates the activation of immune cells hence an important additional mechanism of action (MOA) for antibodies. One MOA occurs when Fc tail region binds to Fc receptors present on effector cells, such as natural killer (NK) cells, macrophages, or neutrophils to facilitate cell lysis. Cell lysis occurs via the release of cytotoxic mediators; antibody-dependent cell-mediated cytotoxicity (ADCC) or via phagocytosis of cells; antibody-dependent cell-mediated phagocytosis (ADCP). In addition, the Fc tail region can activate the complement cascade through binding of C1q which can result in cell lysis by several different mechanisms. These mechanisms include the formation of the membrane attack complex (MAC), that directly induces the lysis of target cells; complement-dependent cytotoxicity (CDC), or the attraction of immune cells through the chemo-attractive activity of the complement components. Furthermore, the opsonisation by complement components identifies the target cells for complement-dependent cell-mediated cytotoxicity (CDCC) by NK cells, macrophages/monocytes and granulocytes, or for complement-dependent cell-mediated phagocytosis (CDCP) by myeloid cells.
What are the antibody isotypes and their functions?
Each different heavy-chain constant region is referred to as an isotype, and the isotype of the heavy chains of a given antibody molecule determines its class. The physical, antigenic, and functional variations between constant regions define the five main classes of heavy chain: M, G, A, E and D. These five classes differ in the sequence and number of constant domains: number of antigen-binding sites and hinge structure. The hinge structure varies in the number and orientation of disulfide bonds leading to variable flexibility between the two Fab arms and between Fab and Fc regions adding distinction to each class. In IgG, IgA and IgD antibody isotypes, the Fc region comprises two identical protein fragments, derived from the second (CH2) and third (CH3) constant domains of the antibody’s two heavy chains. In IgM and IgE, the Fc regions comprise three heavy chain constant domains (CH domains 2-4) in each polypeptide chain.
There are also smaller variations between constant regions that define the subclasses found in varied species. The IgG subclasses are the most varied in mouse models and humans. Human IgG isotypes consist of four subclasses (IgG1, IgG2, IgG3 and IgG4) each containing a different heavy chain. In mice, the IgG isotypes are divided into five sub-classes (IgG1, IgG2A, IgG2B, IgG2C and IgG3). In rats, there are four (IgG1, IgG2A, IgG2B, IgG2C) subclasses. These differences in the Fc region help to mediate the antibody mechanism of action (MOA) through binding to FcRs as indicated in Table 1.
Table 1: Functional Activity of Human Antibody Isotypes
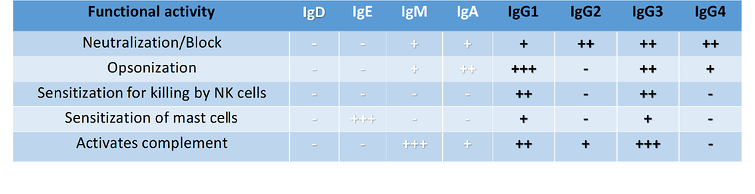
++ moderate functional activity ++++ high functional activity Importance of antibody isotype in therapeutic monoclonal antibodies:
Antibody isotypes for tumor immunotherapy
When developing therapeutic monoclonal antibodies (mAbs), the choice of IgG subclass is especially important and needs to be considered. For example, IgG1 has the maximum potential to activate all 5 effector functions (Table 1), therefore, this isotype may be ideal for eliminating cancer tumours. The antitumor IgG1 antibodies can exert their antitumor activity by blocking growth factor receptors or apoptosis induction. Murine IgG2a is also known for high functional similarity to human IgG1 and is capable of the same Fc-mediated effector function. Whereas the human IgG2 isotype is selected for the neutralisation of antigens. By contrast, IgG3 is seldom used for therapeutic mAbs due to the long hinge region being prone to proteolysis resulting in a decreased half-life. Human IgG4 is distinct from all other IgG subclasses and antibody classes (IgA, IgM, IgE); since the Fc region does not activate complement cascade, but still has other effector functions as indicated in Table 1. Currently, a substantial majority of approved functional grade antibodies in tumour therapy are of human IgG1 isotype. However, human IgG2 and IgG4 isotypes are increasingly used due to their non-Fc-mediated effector functions. An example of IgG4 usage is in as a checkpoint inhibitor mAbs for which ADCC and CDC activities are preferentially avoided. Further engineering of IgG by specific point mutations within IgG revealed greater possibilities to adjust antibody effector functions. For example, some MBLI isotypes are modified with point mutations to stabilise the hinge region of IgG4 or reduce IgG1 Fcγ receptor binding capacity. Thus, it is the combinations of Fab-mediated antigen-specificity and the activation and/or inhibition of Fc region-mediated effects that lead to the outcome of an antibody-based therapy. It is this variety in antibody structure that enables the development of various treatments but demands control of the intended therapeutic effect for in vivo antibody-based studies.
Why are isotype controls important?
When testing antibodies preclinically, you need to control for any variable that might impact the interpretation of study results. The engagement of immune cells via the Fc regions can have an impact on antibody efficacy. A matched isotype will help to determine the engagement of the FC region and its impact on your true drug effects. The main factors to consider when choosing the appropriate isotype control antibody for a study are host, isotype, conjugation, light chain, and concentration. In addition, the formulation of control isotype is a critical factor for in vivo studies. Simple controls such as PBS or avoiding an isotype control can contribute to misinterpretations of data leading to false assumptions of the antibody studies.
Why PBS is not a Suitable Substitute for an Isotype Control
While the use of PBS instead of an isotype control antibody as a negative control is not ideal, it does occur in published research. Using this non-optimal control factor can result in a range of issues during efficacy studies. Negative controls like PBS cannot induce the same effects of the Fc region of an isotype, potentially creating misleading data when compared to the test article.
Use of PBS in Efficacy Experiments Fails to Simulate the Effects Caused by Widespread FcR Engagement
Using PBS as a simplified negative control in a range of in vivo efficacy assays can result in an inaccurate interpretation of results, due to performance differences observed between PBS and the isotype control as illustrated in Figures: 2-4.
Figure 2: Difference in tumor volume with IgG2a isotype control compared to PBS in CT-26. WT syngeneic colon cancer model.
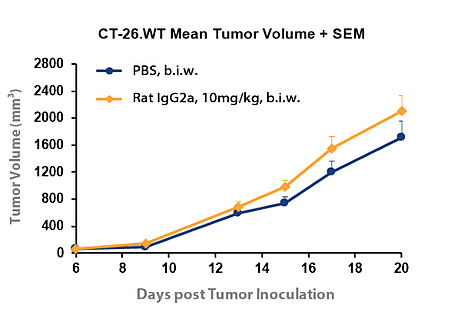
Figure 2 compares growth of the CT-26.WT syngeneic colon cancer model in the presence of PBS or rat IgG2a isotype control. A modest difference was observed between growth rates for the differing control agents. Still this comparison helps to illustrate that the inhibitory effect on tumour growth of a corresponding target antibody must be compared with the matched isotype control and not PBS for a correct calculation of tumour growth inhibition and an accurate measurement of drug efficacy.
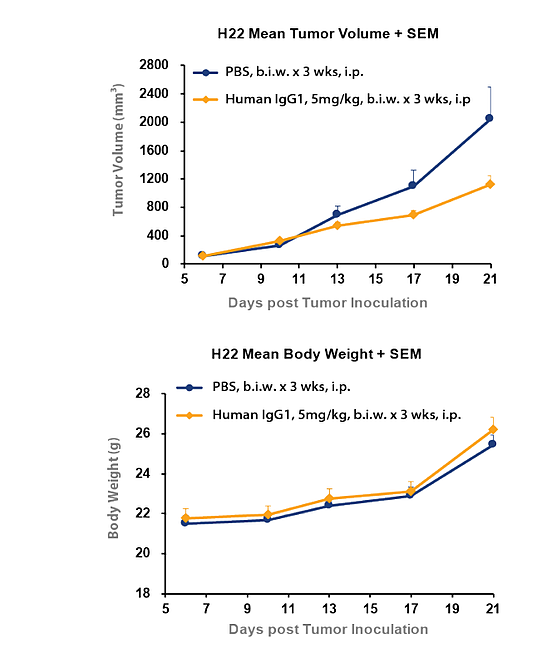
In Figure 3, tumour inhibition was observed with hIgG1 isotype control in H22 syngeneic liver cancer model. This study shows the tumour volume for the human IgG1 isotype was significantly lower when compared to PBS control. This difference clearly illustrates the inhibitory effect on tumour growth of a corresponding target antibody must be compared with the hIgG1 isotype control and not PBS for a correct calculation of tumour growth inhibition and an accurate measurement of drug efficacy.
Figure 3: Tumor inhibition can be observed with hIgG1 isotype control compared to PBS in H22 syngeneic liver cancer model
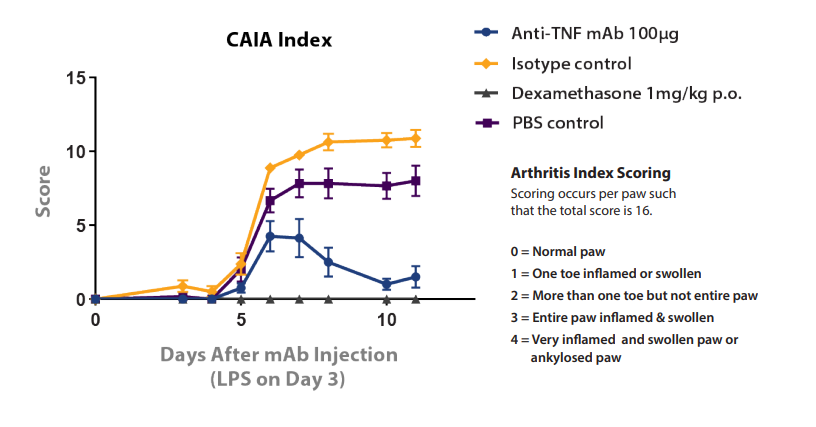
The importance of comparison with the correct control agent is also exemplified in the Collagen antibody-Induced Arthritis (CAIA) model shown in Figure 4. In this model, rheumatoid arthritis in BALB/c mice is induced through the administration of an atherogenic antibody followed by LPS, which can then be treated with potential new agents. The model is treated with dexamethasone (with PBS control) or an anti-TNF mAb (with IgG1 isotype control). The PBS and isotype controls show a clear performance difference, which would lead to an erroneous interpretation of anti-TNF mAb statistics if compared instead with a PBS control.
Figure 4: Performance Difference between PBS and Isotype Negative Controls in CAIA Model
Summary
Therapeutic monoclonal antibodies comprise most of the in-vivo-based testing, with more than 150 trials worldwide and represent the fastest-growing class of new therapeutic molecules. Currently, approved monoclonal antibodies treat autoimmune disorders, non-Hodgkin’s lymphoma, B-cell leukaemia, and other cancers. Testing of mAb in vivo efficacy in immuno-oncology research is critical to determine the different degrees of tumour growth inhibition. As illustrated in Figures 2-4, the comparison of the test antibody needs to be against an isotype control to make sure the antibody effect is not over- or underestimated. Isotype controls are essential tools in a range of in vivo efficacy providing appropriate negative controls for accurate measurement of antibody drug effects. The substitution of other agents such as PBS for isotype controls causes a range of issues which can hinder study performance and contribute to erroneous results. The use of high-quality isotype controls provides a reliable method to accurately differentiate specificity versus background for precision drug development assays.
In vivo grade isotype control antibodies
MBLI’s CrownVivo Isotype Control Antibodies are an ideal reagent choice for your studies. MBLI provides a range of isotype controls suitable for in vivo studies. Functional grade isotype controls* manufactured specifically for in vivo depletion, activation, neutralisation, or blocking studies for human, mouse, or rat models.
References
Tsumoto, Kanta, et al. “Future Perspectives of Therapeutic Monoclonal Antibodies.” Immunotherapy, vol. 11, no. 2, 2019, pp. 119–127., doi:10.2217/imt-2018-0130.
Vonderheide, Robert H., and Martin J. Glennie. “Agonistic CD40 Antibodies and Cancer Therapy.” Clinical Cancer Research, vol. 19, no. 5, 2013, pp. 1035–1043., doi:10.1158/1078-0432.ccr-12-2064.
Originally published by Beata Boczkowska, Ph.D. on Aug 2, 2022 12:07:46 PM at blog.mblintl.com/
Caltag Medsystems is the distributor of MBL products in the UK and Ireland. If you have any questions about these products, please contact us.
