TransFix® (CE/IVD) is a stabilisation solution that stabilises cellular suspensions of both human and animal origin whilst preserving the integrity and antigenicity of leukocytes for transportation and storage. The components of TransFix stabilise cellular markers, allowing analysis by flow cytometry to be carried out at a more convenient time for the user. TransFix can be used in clinical diagnostic testing, clinical trials and a wide range of research applications.
Key Benefits of TransFix
- Extend the window for sample analysis
- Preserve sample integrity during shipment between sites, without the need for cold chain transport
- Reduce false negative results
- Permits sample batching
- Enable repeat testing without requiring patient recall
- Cost savings associated with reduction in reagent usage and requirement for out of hours testing
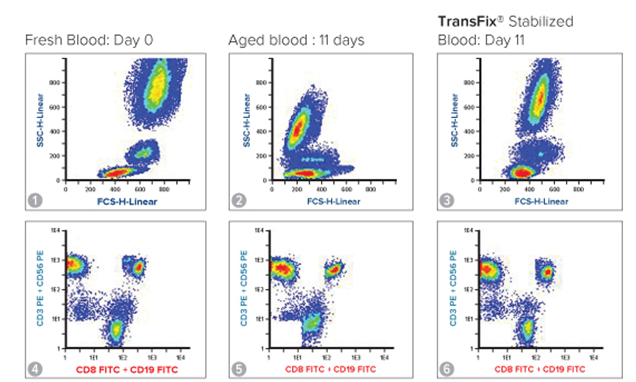
Diagrams 1 & 4 are flow cytometry density dot plots that demonstrate the normal leukocytic immunophenotype seen in a fresh human whole blood sample (analysed on day of collection). Granulocyte, monocyte and lymphocyte populations are all distinct (1), as are T cells, NK cells and B cells (4).
Diagrams 2 & 5 demonstrate the leukocytic immunophenotype in an untreated human whole blood sample after 11 days of storage. Leukocyte subsets – particularly monocytes are less distinct (2), and B cells & NK cells cannot be easily identified (5).
Diagrams 3 & 6 show the leukocytic immunophenotype in TransFix stabilised whole human blood sample after storage for 11 days. All leukocyte subsets, including monocytes, B cells and NK cells can be clearly identified.
Applications of TransFix
- Human Blood Stabilisation
For immunophenotyping, untreated blood samples have a viable shelf life of 24-48 hours due to enzymatic degradation of cellular markers. Treating blood samples with TransFix enables flow cytometric analysis of extracellular markers at 2°C to 25°C for 10 days and at 37°C for 3 days.View publications referencing the use of TransFix for immunophenotyping applications here.
enzymatic degradation of cellular markers. Treating blood samples with TransFix enables flow cytometric analysis of extracellular markers at 2°C to 25°C for 10 days and at 37°C for 3 days.View publications referencing the use of TransFix for immunophenotyping applications here.
- Stabilisation of Cerebrospinal Fluid
TransFix/EDTA CSF Sample Storage Tubes have been developed in collaboration with research oncologists and contain TransFix that has been optimised for leukocyte stabilisation within cerebrospinal fluid (CSF).
 Leukocytes within CSF samples are typically low in numbers and cells often spontaneously disrupt upon removal from the body. Clinical studies have shown that in the time between a sample being collected and analysed, 40% of samples suffer significant cellular disruption rendering them unsuitable for testing. These patient require repeat lumbar puncture until a viable sample is collected. It is therefore critical to stabilize CSF samples immediately upon extraction to prevent cellular disruption.TransFix has been used for CSF stabilisation by a number of leading oncologists. Guidelines published in the British Journal of Haematology recommend that unless immediate analysis is possible then samples should be stored in TransFix for up to 72 hours prior to analysis (Johansson et al, 2014). Research has also shown that lymphocyte yields in general are higher in TransFix treated CSF samples than in fresh samples.Please note that TransFix/EDTA CSF Sample Storage Tubes are currently a Research Use Only product and Cytomark recommends that internal validation of the product must be completed by the end user before considering use of the products in a clinical setting.
Leukocytes within CSF samples are typically low in numbers and cells often spontaneously disrupt upon removal from the body. Clinical studies have shown that in the time between a sample being collected and analysed, 40% of samples suffer significant cellular disruption rendering them unsuitable for testing. These patient require repeat lumbar puncture until a viable sample is collected. It is therefore critical to stabilize CSF samples immediately upon extraction to prevent cellular disruption.TransFix has been used for CSF stabilisation by a number of leading oncologists. Guidelines published in the British Journal of Haematology recommend that unless immediate analysis is possible then samples should be stored in TransFix for up to 72 hours prior to analysis (Johansson et al, 2014). Research has also shown that lymphocyte yields in general are higher in TransFix treated CSF samples than in fresh samples.Please note that TransFix/EDTA CSF Sample Storage Tubes are currently a Research Use Only product and Cytomark recommends that internal validation of the product must be completed by the end user before considering use of the products in a clinical setting.
3. Immunostaining of Circulating Tumour Cells
The number of Circulating Tumour Cells present in patient blood is very low, therefore the usefulness  of CTC assessments depends upon accurate cell counts and the corresponding analysis of molecular targets. Blood samples older than 48 hours are no longer suitable for examination. TransFix stabilised blood samples were used for CTC analysis in an independent study. CTCs were detected by image analytics after fluorescence scanning microscopy. Addition of TransFix to blood samples at the time of collection has been shown to significantly extend the integrity of CTC’s within the samples. Read the case study here.
of CTC assessments depends upon accurate cell counts and the corresponding analysis of molecular targets. Blood samples older than 48 hours are no longer suitable for examination. TransFix stabilised blood samples were used for CTC analysis in an independent study. CTCs were detected by image analytics after fluorescence scanning microscopy. Addition of TransFix to blood samples at the time of collection has been shown to significantly extend the integrity of CTC’s within the samples. Read the case study here.
Cytomark has not independently verified the stabilisation of bone marrow cells using TransFix. Cytomark does not take liability if TransFix is used in applications other than those validated by Cytomark.
4. Stabilisation of Bone Marrow Cells
Independent research involving the use of TransFix to stabilise bone marrow samples has been published, including the use of TransFix for the enumeration of bone marrow mast cells and for immunophenotyping rat bone marrow stem cells.
Cytomark has not independently verified the stabilisation of bone marrow cells using TransFix. Cytomark does not take liability if TransFix is used in applications other than those validated by Cytomark.
5. Stabilisation of Animal Blood
 Like with human whole blood specimens, animal whole blood degrades over time, rendering it no longer suitable for examination by flow cytometry. Researchers have found that the addition of TransFix to animal blood samples at the time of collection has significantly extended the integrity of the samples in several different species.
Like with human whole blood specimens, animal whole blood degrades over time, rendering it no longer suitable for examination by flow cytometry. Researchers have found that the addition of TransFix to animal blood samples at the time of collection has significantly extended the integrity of the samples in several different species.
Independent research studies involving the use of TransFix in stabilising animal blood samples have been published:
Reference 1: Use of TransFix on Chicken Blood Samples
Reference 2: Use of TransFix on Turkey Blood Samles
TransFix has also been used to successfully stabilise whole blood from the following species: mouse, rat, guinea pig, sheep, seal, turtle, ostrich and pig. However, these studies have yet to be published. Cytomark does not take liability if TransFix is used in applications other than those validated by Cytomark.
TransFix Formats Available:
TransFix/EDTA Vacuum Blood Collection Tubes (CE/IVD)
– Recommended for Immunophenotyping/Human Blood Stablilisation
– Stabilises blood at point of collection
– 3ml and 9ml Tubes available
TransFix Sample Storage Tubes (CE/IVD)
– 1.2ml Storage Tubes containing 0.2ml TransFix without EDTA
TransFix/EDTA CSF Sample Storage Tubes (RUO)
– Optimised for CSF stabilisation.
– Internal validation required for use in clinical applications
TransFix Reagent (CE/IVD)
– Available in 1ml or 20ml stock volumes
– Please contact us if you require alternative volumes of TransFix
TransFix is manufactured by Cytomark – a division of Caltag Medsystems. If you would like further information about TransFix, please do not hesitate to contact us at cytomark@caltagmedsystems.co.uk, or telephone (+44) 01280 827460.