Though the adaptive immune system functions similarly across most vertebrates, some species such as bovine, camelids, and sharks stand out from the others with unique antibodies that only occur in their own evolutionary path. These antibodies have characteristics that can help researchers target epitopes that would otherwise be inaccessible.
In addition to ProSci‘s standard animals for antibody production, ProSci offers custom antibody production for other species upon customer’s request.
Bovine Antibodies
 Bovine antibodies are unique because they have a very long CDR3 binding loop comprised of over 60 amino acids. The CDR3 domain is part of the antigen binding region of an antibody that gives an antibody its specificity and allows for antibody diversity. This long CDR3 loop allows bovine antibodies to bind epitopes inaccessible to antibodies from other species, such as crevices and pores. For a more detailed picture of these unique antibodies, check out this article about Vaughn Smider’s work at The Scripps Research Institute that lead to the discovery of these novel antibodies. If you are interested in learning more about developing bovine antibodies, contact ProSci here.
Bovine antibodies are unique because they have a very long CDR3 binding loop comprised of over 60 amino acids. The CDR3 domain is part of the antigen binding region of an antibody that gives an antibody its specificity and allows for antibody diversity. This long CDR3 loop allows bovine antibodies to bind epitopes inaccessible to antibodies from other species, such as crevices and pores. For a more detailed picture of these unique antibodies, check out this article about Vaughn Smider’s work at The Scripps Research Institute that lead to the discovery of these novel antibodies. If you are interested in learning more about developing bovine antibodies, contact ProSci here.
Camelid Antibodies
Camelids, such as llamas, alpacas, and camels, have a unique type of antibody called a heavy chain only antibody (hcAb). These hcAbs lack the light chain variable domain that is common to most antibody isotypes, making the overall structure of the antibody smaller and allowing it to bind in more obscured  epitopes. Additionally, these hcAbs can potentially have extended CDR3 domains which increase variability. This longer CDR3 can be stabilized by an extra disulphide bond, helping to stabilize the overall structure. Lastly, the N-terminal portion of CDR1 is more variable as well, allowing for additional antibody diversity (Vu et al. 1997). It is important to note that camelids still produce typical IgG antibodies as well as several other isotypes, and that only the hcAbs have these characteristics, and must be isolated using unique protocols.
epitopes. Additionally, these hcAbs can potentially have extended CDR3 domains which increase variability. This longer CDR3 can be stabilized by an extra disulphide bond, helping to stabilize the overall structure. Lastly, the N-terminal portion of CDR1 is more variable as well, allowing for additional antibody diversity (Vu et al. 1997). It is important to note that camelids still produce typical IgG antibodies as well as several other isotypes, and that only the hcAbs have these characteristics, and must be isolated using unique protocols.
Through techniques like phage display, the sequence of heavy chain only antibodies can be manipulated to isolate the binding domain, which results in variable fragments of heavy chain antibodies (VHHs), also called a single domain antibody. Click to read more about single domain antibodies.
Shark Antibodies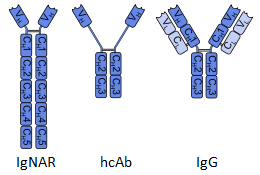
Shark and cartilaginous fish antibodies are unique because they also possess the extended CDR3 loop that camelids and bovines have and are also similar to the camelid antibody in that they also produce heavy chain only antibodies. However, they differ in that the constant region of their antibodies is much longer than that of the camelid hcAbs. These long constant domain antibodies are called IgNARs (immunoglobulin new antigen receptor) and are thought to serve a similar purpose to IgM antibodies in humans for detecting new antigens.
Evolutionarily, it’s thought that these special shark IgNAR antibodies have these extra domains in order to remain stable in their bloodstream. Shark blood has a high concentration of urea in order to maintain a similar osmolarity to seawater, but urea denatures proteins very easily. This paper explains that the extra constant domains help to preserve the robust shark IgNAR structure with additional disulphide bonding.
An Honorable Mention to Green Turtles
In October of 2015, a paper was published on green turtle antibodies. These turtles have asymmetrical IgY antibodies that have never been seen before in other species. For more information, take a look at the October 2015 Antibody Update, or read the paper from the Journal of Immunology.
Find out how ProSci can help your Antibody needs today!
