FETALtrol™ is a 3-level control set that can be used to validate and monitor the quality of both flow cytometric and Kleihauer-Betke manual procedures for the detection of Foetomaternal haemorrhage (FMH). As a result, use of FETALtrol eliminates the need to acquire cord blood for the production of home brew controls, saving time precious to the laboratory workforce.
FETALtrol™ is a 3-level control set that can be used to validate and monitor the quality of both flow cytometric and Kleihauer-Betke manual procedures for the detection of Foetomaternal haemorrhage (FMH). As a result, use of FETALtrol eliminates the need to acquire cord blood for the production of home brew controls, saving time precious to the laboratory workforce.
Key Features:
- Eliminates the need to acquire cord blood to make home brew controls, saving time precious to the laboratory workforce
- Three validated levels which correspond to clinical decision points for anti-D therapy
- FDA cleared as a hematologic control for fetal red cell detection
- IVD / CE registered
- 3 months total shelf life for unopened vials
 Applications
Applications
- Flow cytometric methods
- Kleihauer-Betke tests
- EQA programs
Product Information
FETALtrol is available in two sizes:
3 Levels (1 x 2 ml vial per level)
Product Code: FH102
Regulatory Status: CE/IVD
3 Levels (2 x 2ml vials per level)
Product Code: FH101
Regulatory Status: CE/IVD
Alert List and Standing Orders
Each batch of FETALtrol™ has a shelf life of 3 months, and is manufactured at the start of every quarter (January, April, July, and October).
To avoid disappointment, we recommend placing a standing order before the new batch becomes available, as only a small amount of stock is manufactured at a time and is sold on a “first come- first served” basis. We also have an email alert list to remind customers when a new batch is due to be released.
If you would like to speak to us about setting up a standing order, or to add your email to our email alert list, please contact us at techsupport@caltagmedsystems.co.uk or by telephone +44 (0)1280 827460.
Principle of FETALtrol™
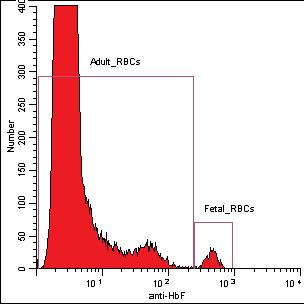
The laboratory determination of the level of foetal cells in maternal circulation remains an important support in the obstetrical management of women with suspected uterine trauma and in the proper dose administration of Rh immune globulin. Limitations in the sensitivity and precision of the widely used manual Kleihauer-Betke method have prompted can increased utilization of flow cytometric methods for fetal cell detection in maternal blood samples.
The anti-HbF flow cytometric method for detection of fetal cells offers a simple, reliable and more precise alternative to the KB manual technique for the assessment of foetomaternal hemorrhage. The method has additional potential applications for the study of HbF levels or frequency of adult red cells with low levels of HbF (F cells) in individuals with haemoglobinopathies.
Figure 1: Results of the high level of FETAL™trol.
Background Information
Detection and quantification of foetal red blood cells (RBCs) in maternal blood samples is essential for obstetrical management. Measurement of foetal RBCs is critical as the extent of Foetomaternal Hemorrhage (FMH), the transplacental passage of foetal RBCs into the maternal circulation, has consequences for further treatment of mother and child.
Frequency and size of FMH is directly influenced by complications in abdominal trauma, suspected placental injury or after a caesarean section. Severe FMH may lead to intra-uterine death. In case of antigen incompatibility between mother and child FMH may result in respiratory problems or anaemia, like Haemolytic Disease of the Newborn.
Also Available: FMH Diagnostic Kits
Foetal Cell Count™ Kit: A complete assay for the routine diagnosis of foetomaternal haemorrhage using anti-HbF and anti-CA.
FMH QuikQuant™ – A rapid assay for foetomaternal haemorrhage quantification (previously supplied by Trillium Diagnostics) by measuring HbF expression.
FETALtrol by IQ Products (previously supplied by Trillium Diagnostics) is distributed in the UK & ROI by Caltag Medsystems. If you would like further information about any of their products, please contact us at techsupport@caltagmedsystems.co.uk or by telephone 01280 827460.