Engineered base editor with near-zero off-target effects
AccuBase® is a cytosine base editor that converts a C•G base pair into a T•A base pair in the genome. It is independently designed by Base Therapeutics and manufactured for global sales by KACTUS. It creatively embeds the deaminase inside the Cas protein to prevent random tethering of deaminase to non-target site, significantly reducing off-target occurrence while still maintaining high editing efficiency.
Leveraging their SAMS™ protein engineering and expression platform, KACTUS has developed a large-scale production process for AccuBase® base editor. They have focused on maximising the stability, purity, and activity of their GMP-grade AccuBase®, ensuring it meets the highest quality standards in compliance with cGMP raw materials for CGT drug manufacturing.
How does AccuBase® work?
Precision editing by activating only upon accurate target binding
After forming a ribonucleoprotein (RNP) with sgRNA, the AccuBase® protein remains in a non-editing state before binding to the target DNA. The deaminase is encapsulated inside the Cas9n protein and does not interact with any non-target DNA, thus significantly reduces the risk of off-target effects. When the RNP binds to the target DNA, the conformation of AccuBase® changes, leading to the exposure of the deaminase domain and effectively editing bases within the 3-12 window range of the target site (with the first position being the farthest from the PAM) (Figure 1).
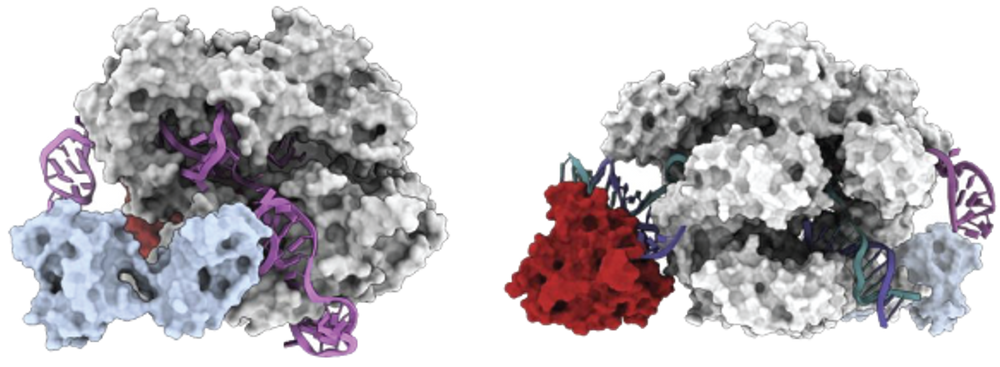
Mechanism of action
The mechanism of action of AccuBase® involves the precise editing of target DNA sequences within the 3-12 base window of the target site, leading to specific point mutations (C to T, G to A) or gene knockouts through the introduction of stop codons or alteration of splicing sites (Figure 2).
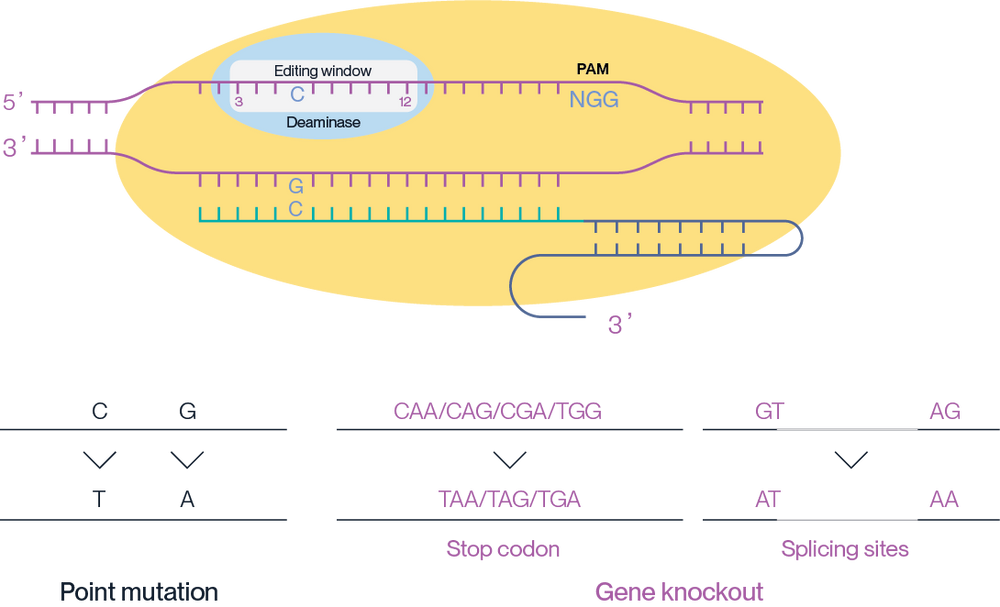
AccuBase® is ideally suited for correcting a single point mutation or for gene knockouts by introducing premature stop codons or inducing exon skipping by disrupting splice acceptor (SA) sites or splice donor (SD) sites.
Product Performance Data
Near-zero off-target effects
High editing activity
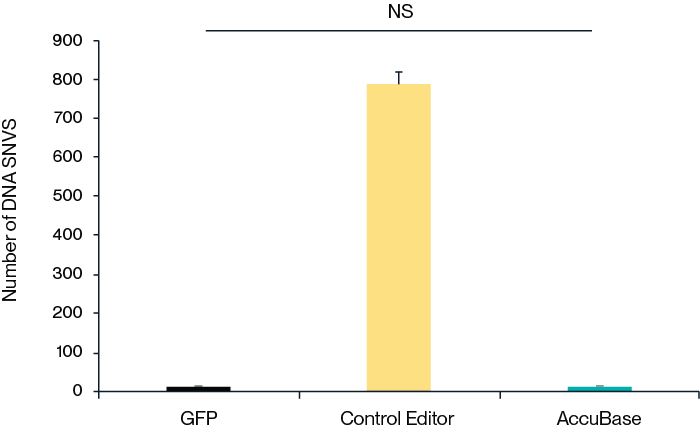
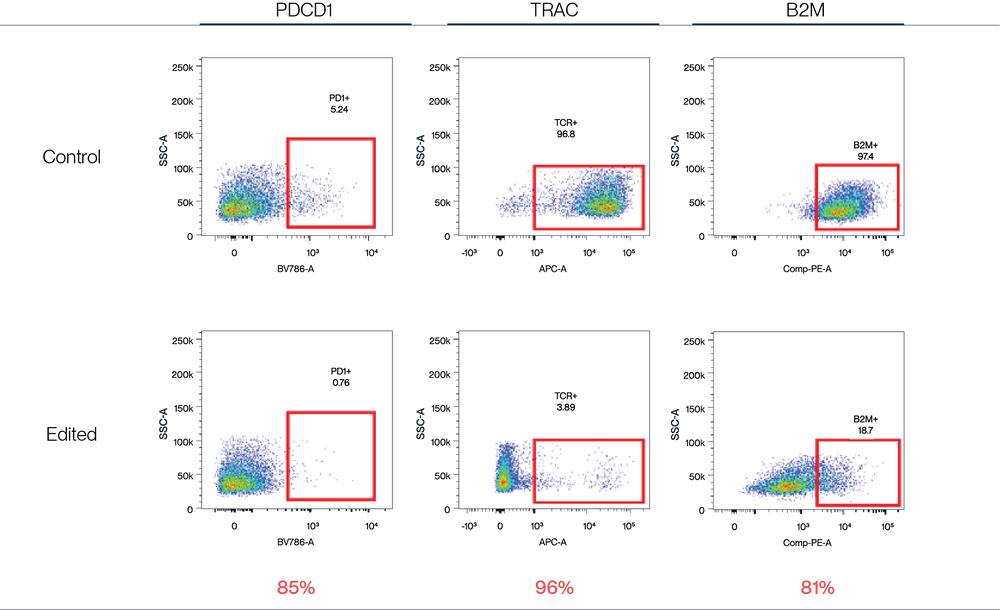
Figure 3. Measurement of off-target effects by GOTI. By leveraging the GOTI (genome-wide off-target analysis by two-cell embryo injection) to measure the off-target effects throughout the whole genome, it was shown that compared to the control base editor (with 700 SNVs detected), the number of SNV obtained after editing by AccuBase® is similar to the GFP (negative control) group, suggesting a near-to-zero off-target effects of AccuBase®.
Figure 4. AccuBase™ RNP was electroporated into activated primary T cells. According to flow cytometry, AccuBase™ can efficiently knock out PD1, B2M, and TRAC proteins on the membrane of activated primary T cells at the protein level. For PD1 and B2M, the knockout efficiency exceeded 80%, while the knockout efficiency of TRAC reached 96%. FACS efficacy % = (positive control – KO group)/positive control* 100%.
Avoids INDEL creation
Avoids chromosomal translocation
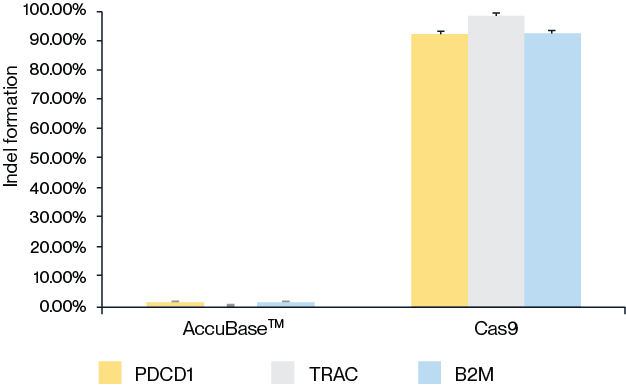
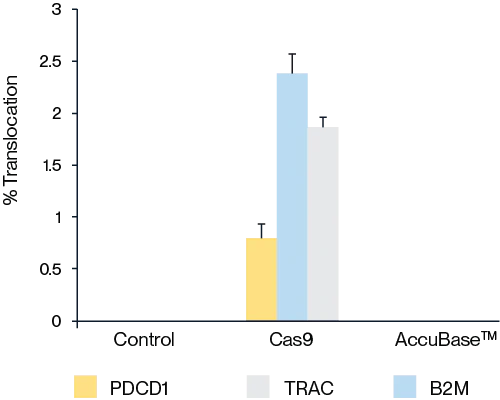
Figure 5. Compared with Cas9 protein, AccuBase™ does not produce DNA horizontal insertions and deletions (INDELs).
Figure 6. Compared with Cas9 protein, AccuBase® does not cause chromosomal translocation.
Product Features
KACTUS offers both GMP-grade and research-grade AccuBase® as off-the-shelf catalog products. Their GMP AccuBase® Base Editor is free from animal-derived components, has greater than 80% purity, and comes with comprehensive quality control documentation as well as additional batch production records upon request. KACTUS are dedicated to supporting your applications of AccuBase® from preclinical research through commercial manufacturing, backed by our robust technical and quality support.
Learn more about their GMP quality management systems →.

Manufactured in a GMP-compliant facility
Raw materials free from animal-derived components
High purity, activity, and stability
Traceable documentation for regulatory support
Off-the-shelf catalog product
Product Specifications & Quality
Product Specifications
Both their GMP-grade and research-grade AccuBase® adhere to the following product specifications:
| Parameter | Specification |
|---|---|
| Express System | E. Coli |
| Concentration | 10mg/ml |
| Molecular Weight | 210.14kDa |
| Form | Liquid |
| Storage Buffer | 30 mM Tris, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 50% Glycerol, pH8.0 |
| Storage | Store at -80 ±10°C |
Quality Control Criteria
Their GMP-grade AccuBase® meets the following quality control criteria:
| Assay | Acceptance Criteria |
|---|---|
| pH | 8.0±0.5 |
| Concentration | 9.0-11.0mg/mL |
| Purity (electrophoresis) | ≥ 80.0% |
| Purity (RP-HPLC) | ≥ 88.0% |
| Purity (SEC-HPLC) | ≥ 80.0% |
| Residual DNase | Sample/Control ≤ 3.0 |
| Residual RNase | Sample/Control ≤ 3.0 |
| Residual Host Cell Protein | ≤ 100.0ng/mL |
| Residual Host Cell DNA | ≤ 200.0ng/mL |
| Endotoxin | ≤ 10.0EU/mg |
| Sterility | Negative |
| Mycoplasma | Negative |
GMP Compliance
GMP-Grade AccuBase® is produced following cGMP standards, ensuring traceability of raw materials, manufacturing processes, and QC release testing. The GMP-Grade AccuBase® is available in long-term bulk supply with high batch-to-batch consistency to ensure suitability for industrial applications. We also offer a research-grade version for a seamless transition from preclinical study to clinical/commercial CGT drug manufacturing.
Documentation Package
GMP-Grade AccuBase® comes with customisable regulatory documentation including Data Sheet, MSDS, COA, TSE/BSE statement, CoO. KACTUS has an integrative documentation system consisting of a digital quality management system, specification program, criteria support documents, and quality reports. The documentation package is customisable based on relevant regulatory filings.
Quality Management System
- ISO13485 Accreditation
- Digital Manufacturing Execution System (MES)
- Process and Analytical method validation
- Batch-to-batch stability and consistency
- Free from antibiotic residues and raw materials of animal origin
- Comprehensive records for batch production
- Pharmaceutical Class A & C Clean Room
- Validated and maintained equipment
Learn more about KACTUS’ GMP production →
AccuBase® ELISA kit
KACTUS has developed an AccuBase® ELISA kit for detection of residual AccuBase® in samples. The kit uses monoclonal antibodies in a sandwich ELISA and demonstrates high accuracy.
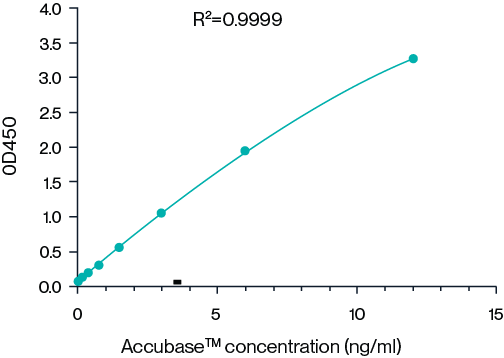
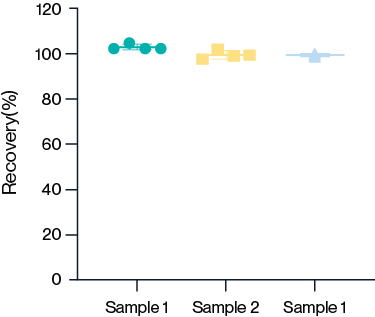
Frequently Asked Questions
Product Specifications
Is AccuBase® manufactured according to GMP guidelines?
KACTUS’ GMP-Grade AccuBase® (#GMP-KD-0001) is always manufactured according to cGMP guidelines. They also offer a research-grade version of AccuBase® (#KD-0001) that does not undergo GMP quality control testing.
What regulatory documentation is available for GMP-Grade AccuBase®?
AccuBase®, GMP-Grade (#GMP-KD-0001) comes with customisable regulatory documentation including Data Sheet, MSDS, COA, TSE/BSE statement, and CoO. Batch production records, batch inspection records, etc, can also be provided. Please contact us so we can learn more about your specific regulatory filings.
What are the advantages of AccuBase® over other gene editing enzymes?
Compared to Cas9 and other Cas family gene editing enzymes, AccuBase® does not cause DNA double-strand breaks, does not require donor DNA, and is not dependent on the cell cycle for precise gene repair. It also does not produce INDELs and thus poses no risk of chromosomal translocations.
Compared to other cytosine base editors, its unique structural design allows editing only at the target site, with no random off-target activity, while maintaining high editing efficiency.
How does AccuBase® have near-zero off-target characteristics?
AccuBase® is an engineered DNA cytosine base editing protein, creatively embedding a deaminase within the Cas protein. When AccuBase® protein forms an RNP with sgRNA, it remains in a non-editing state until it binds with the target DNA. The deaminase, encased inside the Cas9n protein, does not act on any non-target DNA, significantly reducing off-target risk. When AccuBase® RNP binds to the target DNA, a conformational change in AccuBase® exposes the deaminase domain, allowing C-to-T editing within the 3-12 window range (with the first position being far from PAM).
What is the difference between research-grade and GMP-grade AccuBase®?
Production Environment: GMP-grade AccuBase® is produced in their GMP manufacturing facilities, whereas research-grade is produced in non-GMP facilities.
Quality Control Testing: GMP-grade AccuBase® includes tests for appearance, concentration, purity (2100), purity (SEC-HPLC), purity (RP-HPLC), endotoxin, DNA enzyme residue, RNA enzyme residue, host protein residue, host DNA residue, sterility, and mycoplasma. Research-grade includes concentration, identification (Bis-Tris PAGE), purity (SEC-HPLC), and endotoxin.
Regulatory Documentation: GMP-grade AccuBase® includes complete method validation, process validation reports, COA, COO, non-animal source statements, stability studies, etc., supporting client project applications. Research-grade includes instruction manuals and COA, not supporting client project applications.
Both research-grade and GMP-grade have the same bacterial strain origin, same materials used in production, same production process, and consistent protein activity.
Product Usage
What are the applications of AccuBase®?
Gene Knockout: Through mutating start codons, introducing stop codons, and altering splice sites.
Gene Repair: Repairing pathogenic sites. Suitable for cells that can be electroporated or directly injected, including adherent cells, suspension cells, embryonic cells (fish, mice, etc.), and plant embryos (gene gun method).
Can AccuBase® achieve multi-gene knockout?
AccuBase® can edit up-to three loci per current data. Please note, the overall editing efficiency will decrease for multi-gene knockout/editing.
How do I design sgRNA for AccuBase®?
The single guide RNA (sgRNA) scaffold for AccuBase® is the same as that used for spCas9. Designing sgRNA for gene knockout with AccuBase® involves considering editing within the 3-12 position window. A simple approach is to first search for triplet codons CAA (Gln), CAG (Gln), CGA (Arg), or TGG (Trp), and then look for suitable PAMs (NGG) nearby.
Are there patent licensing issues with AccuBase®?
AccuBase®, designed and developed by Base Biotechnology, holds full independent intellectual property rights and foundational technology patents. Potential partners/clients interested in using AccuBase® in their projects need to discuss patent licensing matters with Base Biotechnology. The company adopts an open, flexible, and sincere approach in negotiations.
How do I use AccuBase®?
For detailed operation instructions, refer to the datasheet. For specific scientific support, please contact us.
Explore AccuBase® Products & Resources
Caltag Medsystems is the distributor of KACTUS products in the UK and Ireland. If you have any questions about these products, please contact us.