Introduction
Chimeric Antigen Receptor (CAR) T cell therapy is a revolutionary approach in the field of cancer immunotherapy. This innovative treatment involves genetically engineering a patient’s own T cells to express chimeric antigen receptors, which are artificial receptors designed to target specific cancer-associated antigens. CAR-T cells combine the antigen recognition capability of antibodies with the potent cytotoxic activity of T cells, enabling them to recognise and eliminate cancer cells with remarkable precision. Over the past decade, CAR-T therapy has demonstrated exceptional success in treating certain hematologic malignancies, such as acute lymphoblastic leukaemia and non-Hodgkin lymphoma. The ability to reprogram a patient’s own immune cells to effectively combat cancer marks a paradigm shift in cancer treatment strategies, offering new hope for patients with otherwise refractory or relapsed diseases. As research continues to advance, CAR-T therapy holds promise for broader applications and improvements, paving the way for a new era of personalised and targeted cancer therapies.
CAR Structure
Chimeric antigen receptor (CAR) consists of three parts: an extracellular domain, a transmembrane domain, and an intracellular signalling domain.
- The extracellular domain contains a single-chain variable fragment (scFv) molecule derived from an antibody to recognise specific cancer cell antigens and a hinge region provides flexibility to the receptor, aiding in optimal antigen binding.
- The transmembrane domain anchors the CAR within the T cell’s membrane, ensuring stability.
- The intracellular signalling domain is composed of one or more costimulatory molecules, such as a cluster of differentiation CD28 and 4-1BB, and a stimulatory molecule, CD3 ζ, transmitting activation signals to the T cell’s interior, initiating a robust immune response against targeted cancer cells.
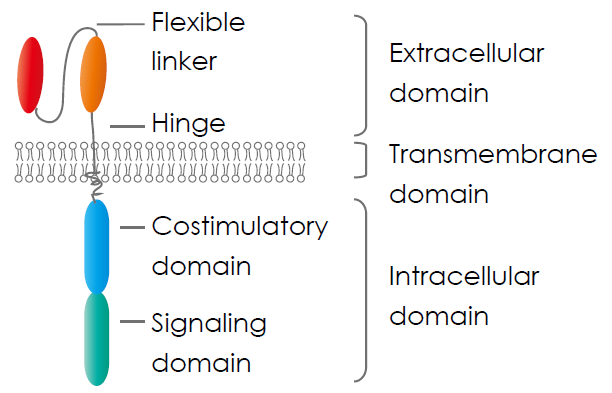
Xin et al., (2022)
CAR Evolution
There are five generations of chimeric antigen receptors (CARs), each building upon the previous to enhance CAR-T cell therapy’s effectiveness.
- The first generation only contained the CD3ζ derived signalling modules. While functional, these CARs had limited efficacy and persistence.
- The second generation contained CD3ζ and a costimulatory molecular domain. like CD28 or 4-1BB, alongside the signalling domain. These additions improved CAR-T cell activation, proliferation, and persistence, resulting in more potent anti-cancer responses.
- The third generation contained CD3ζ and multiple costimulatory molecular domains, including CD28, 4 1BB, OX40 or ICOS, etc, aiming to amplify T cell activity.
- The fourth generation is called TRUCKs (T cells redirected for antigen-unrestricted cytokine-initiated killing), engineered to secrete immune-stimulating molecules upon antigen recognition. This design aims to recruit and activate other immune cells against the tumour.
- The fifth generation included simultaneous activation of TCR, costimulatory molecular domain, and cytokine triple signalling to stimulate cell proliferation and enhance its persistence.
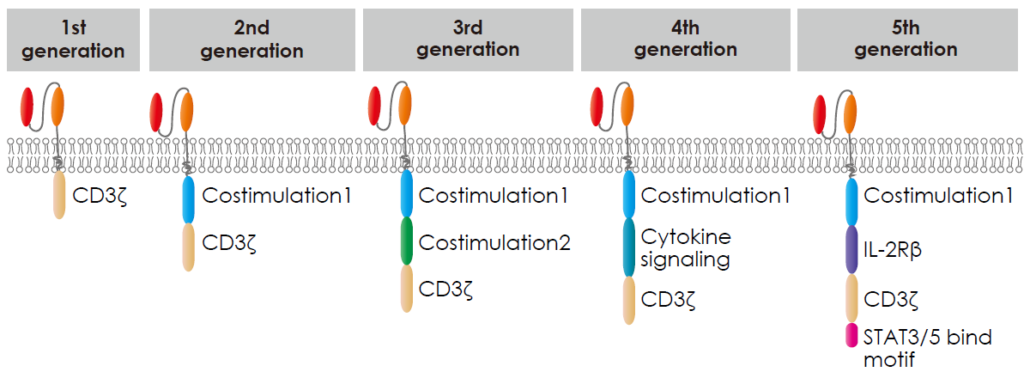
Xin et al., (2022)
In-Vitro CAR-T Cell
In this method, T cells are extracted from the patient and engineered outside the body. The extracted T cells are modified to express the CARs, then expanded and cultured to create a large population of CAR-T cells. Once their numbers are sufficient, these cells are infused back into the patient. In-Vitro CAR-T allows for precise CAR design, rigorous quality control, and optimal expansion of modified cells before treatment, but it involves complex cell manipulation processes.
Six FDA Approved CAR-T Cell Products
| Product Name | Targets | Target Diseases | Time of Approval |
|---|---|---|---|
| Kymriah® (tisagenlecleucel) | CD19 | B-ALL ; DLBCL | August 2017 |
| Yescarta® (axicabtagene ciloleucel) | CD19 | DLBCL ; RRFL | October 2017 |
| Tecartus® (brexucabtagene autoleucal) | CD19 | R/R MCL | July 2020 |
| Breyanzi® (lisocabtagene maraleucel) | CD19 | DLBCL | February 2021 |
| Abecma® (idecabtagene vicleucel) | BCMA | R/R MM | March 2021 |
| Carvykti® (ciltacabtagene autoleucel) | BCMA | R/R MM | February 2022 |
Chen et al., (2022)
B-ALL, B-cell acute lymphoblastic leukaemia; DLBCL, Diffuse large B-cell lymphoma; R/R FL, relapsed or refractory follicular lymphoma; R/R MCL, relapsed or refractory mantle cell lymphoma; R/R MM, relapsed or refractory multiple myeloma.
In-Vivo CAR-T Cell
In this approach, CAR-T cells induced by nanocarriers loaded with CAR genes and gene editing tools are generated within the patient’s body. Once inside the body, these modified T cells start expressing the CAR receptor and target cancer cells. In-Vivo CAR-T offers simplicity and avoids the need for cell collection, engineering, and reinfusion, but controlling the modification process can be challenging.
In-Vivo CAR-T Cell Gene Delivery Systems
| Viral Vectors |
|---|
| Viral vectors have good transfection efficiency and are widely used to deliver genes in various applications, but they suffer from immunogenicity and cellular toxicity. Example: Lentiviral vector (LV) and Adeno-associated virus vector (AAV) |
| Non-Viral Delivery Systems |
|---|
| Physical delivery systems have low immunogenicity but cannot target internal organs. Example: electroporation, needle injection, laser irradiation, and gene guns. |
| Chemical delivery systems have recently gained worldwide attention. Lipid-based nanoparticles are one of the most attractive non-viral vectors for gene delivery as several formulations of these carriers have been approved to use in the clinic. Some T cell-targeted lipid nanoparticles (LNPs) with plasmid DNA or in vitro-transcribed (IVT) mRNA have been reported. Example: cationic lipids or polymer-based nanoparticles, gold nanoparticles, silica nanoparticles, and exosomes. |
Wakao et al., (2023)
Challenges & Potential Strategies in CAR-T Cell Therapy
| Limitations of CAR-T Cell Therapy | Potential Strategies |
|---|---|
| Antigen escape | – Targeting multiple antigens (dual or tandem CARs) |
| On-target off-tumour effects | – Targeting tumour-restricted post-translational modifications |
| CAR-T cell trafficking and tumoir infiltration | – Local administration vs systemic delivery – Engineering CAR-T cells to enhance penetration through physical barriers (tumour stroma) |
| Immunosuppressive microenvironment | – Combination immunotherapy with CAR-T cells and checkpoint blockade – Engineering CAR-T cells to provide immunostimulatory signals in the form of cytokines or CARs resistant to immunosuppressive factors. |
| CAR-T cell-associated toxicities | – Altering CAR structure to ameliorate toxicity – Modifying CAR transduced T cells and neurotoxicity – CAR “off-switches” |
Sterner et al., (2021)
Originally posted by Abnova on https://www.abnova.com/en-global/newsletter/newsletter/content/newsletter2023-09-01
Caltag Medsystems is the distributor of Abnova products in the UK and Ireland. If you have any questions about these products, please contact us.
