- Principle of the cell-based T2 shift assay and the cell-free QuickSwitch™ Quant assay
- Comparison of the cell-based T2 shift assay and the cell-free QuickSwitch™ Quant assay
Immuno-oncology is a growing field in which different strategies such as immune modulators, vaccines and adoptive cell therapies are applied for new immunotherapies. Cancer vaccine immunogenicity and stability are two important parameters in vaccine development. In addition, vaccine immunogenicity implies accurate enumeration of antigen specific T-cells. MHC tetramers can help monitor peptide vaccine immunogenicity and stability.
T-cell receptors (TCR) recognize a unique MHC-peptide combination. The half-life of the MHC-Peptide complex on the cell surface is proportional to the peptide affinity with the MHC molecule. Peptide exchange occurs in vivo during biosynthesis of MHC class I molecules. The QuickSwitch™ principle is based on this system. The QuickSwitch™ platform allows determining peptide binding to MHC molecules. Moreover, the percentage of peptide exchange can be calculated. Evaluating the biological activity of a cancer vaccine is thus an important application of this kit.
To illustrate this application the DepoVax™–based ovarian cancer vaccine candidate, DPX-Survivac, is used. The vaccine consists of a mixture of liposomes and different peptides, covering different MHC alleles. Peptides, encapsulated by liposomes, are recognized by antigen presenting cells (APC) and are transferred to the cell surface by MHC molecules. T-lymphocytes bind to the APC cells in an MHC/peptide dependent manner and are activated, proliferate, and subsequently kill tumor cells that display the same MHC/peptide complexes as the APCs.
Download AAI QuickSwitch™ poster.
Principle of the cell-based T2 shift assay and the cell-free QuickSwitch™ Quant assay
T2 shift assay
T2 cells are a human cell line that express low levels of HLA-A2 on their surface due to deficiency in TAP protein. Upon incubation with a peptide that can bind to HLA-A2, expression of HLA-A2 on the surface increases as the peptide stabilizes the complex. After antigen stimulation, the relative HLA-A2 expression in vitro is quantified by immunofluorescent staining of T2 cells with a fluorescein-conjugated monoclonal antibody (anti-HLA-A2).
QuickSwitch™ Quant Tetramer assay
The kit includes a tetramer with an irrelevant, low affinity binding exchangeable peptide, a peptide exchange factor, HLA capture beads and a FITC conjugated antibody, which recognizes the exiting peptide.
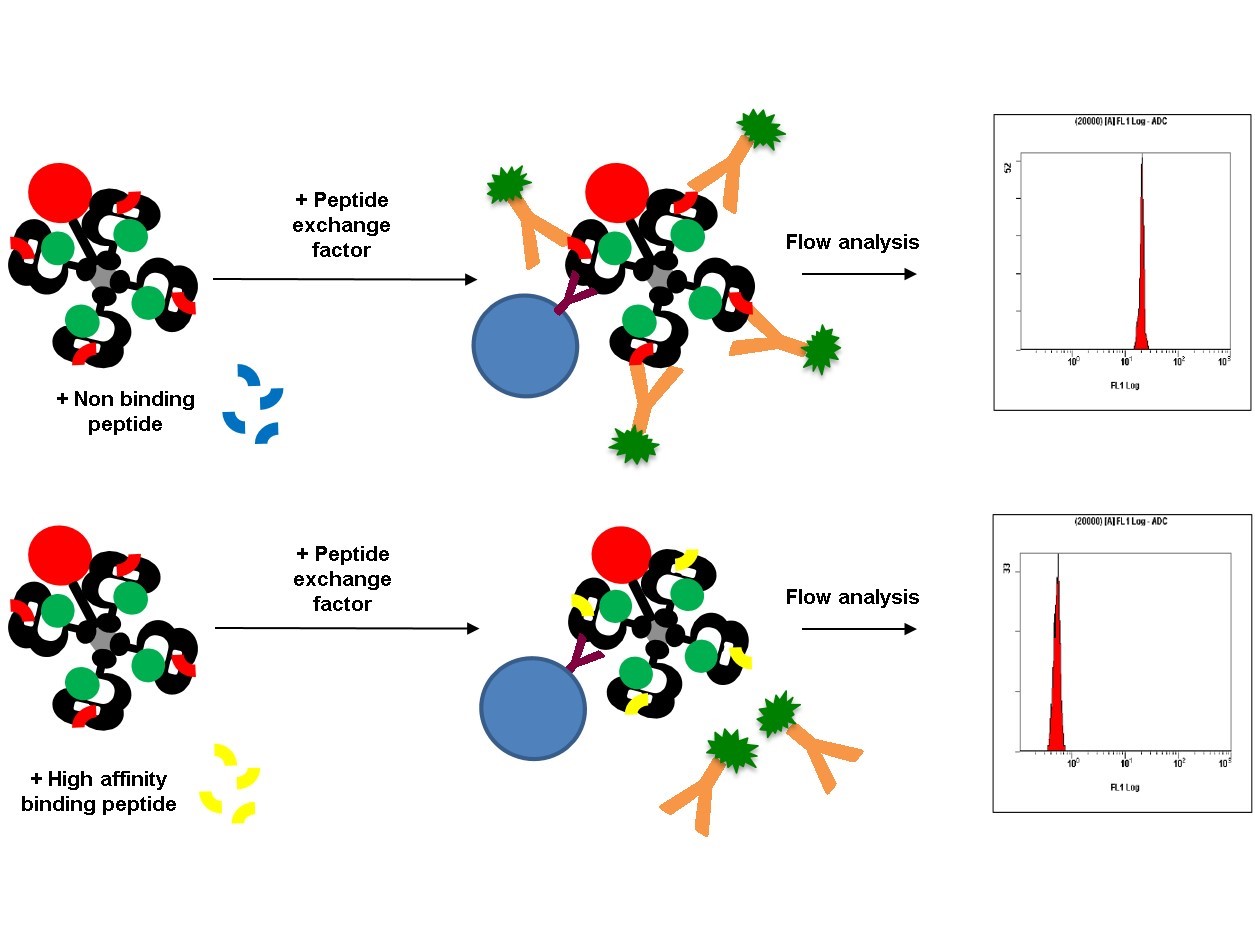
In this study the SurA2.M peptide with the following sequence, LMLGEFLKL, is used. The predicted (IEDB artificial neural network method) HLA-A2 affinity of the peptide is 25,41 nM. Do you want to know more about the materials and methods, have a look at our AAI QuickSwitch™ poster.
Comparison of the cell-based T2 shift assay and the cell-free QuickSwitch™ Quant assay
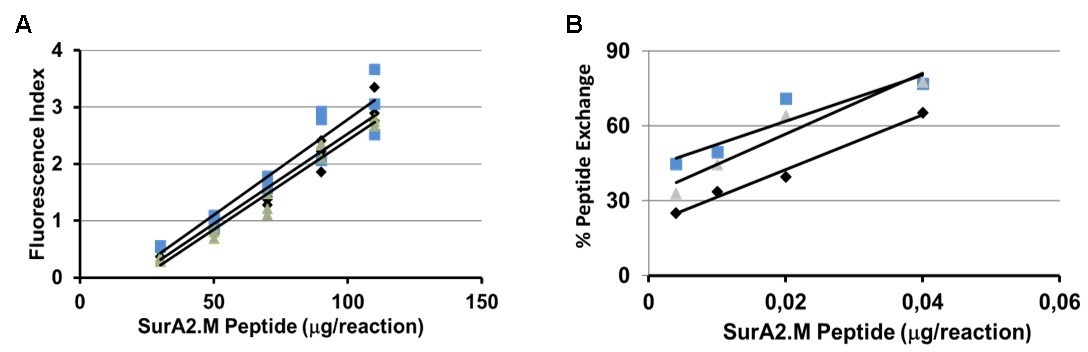
We could find that the HLA-A2 QuickSwitch™ Quant peptide exchange assay can be used to evaluate the biological activity of peptide-based vaccines. Similar to the T2 shift assay, QuickSwitch™ quantifies HLA-A2/peptide interactions, but does this in a cell-free environment. Sensitivity of the HLA-A2 QuickSwitch™ Quant is at least 200x higher than the HLA-A2 T2 shift assay. The HLA-A2 QuickSwitch™ Quant peptide exchange assay is user-friendly, fast and can be performed in a high-throughput 96-well plate format.
MBL products are distributed In the UK and Ireland by Caltag Medsystems. If you have any questions about the products in this blog, please contact us.

