A familiar protocol for easy execution.
KACTUS’ AAV Titration ELISA kits use a standard sandwich ELISA method, utilising exclusive AAV antibodies developed using their proprietary immunisation scheme.
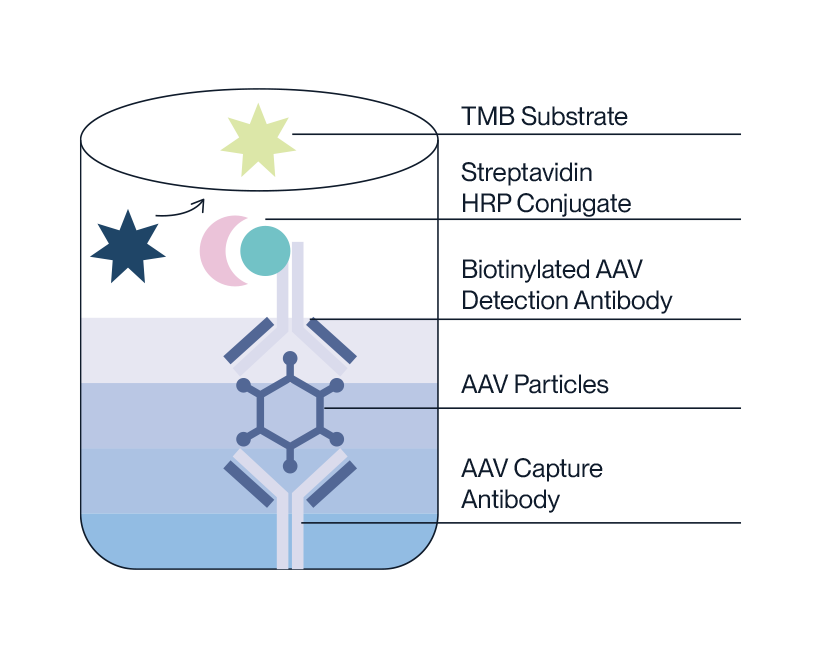
- Pre-coated microtiter plate with removable single-use test strips.
- AAV standard and sample is incubated on plate.
- Biotinylated detection antibody is added to bind AAV capsids.
- Streptavidin HRP conjugate is added to bind with antibody-AAV complex.
- HRP will then oxidise TMB substrate to produce a colour change proportional to AAV capsid titer.
Proprietary monoclonal antibodies developed using enhanced immunisation scheme.
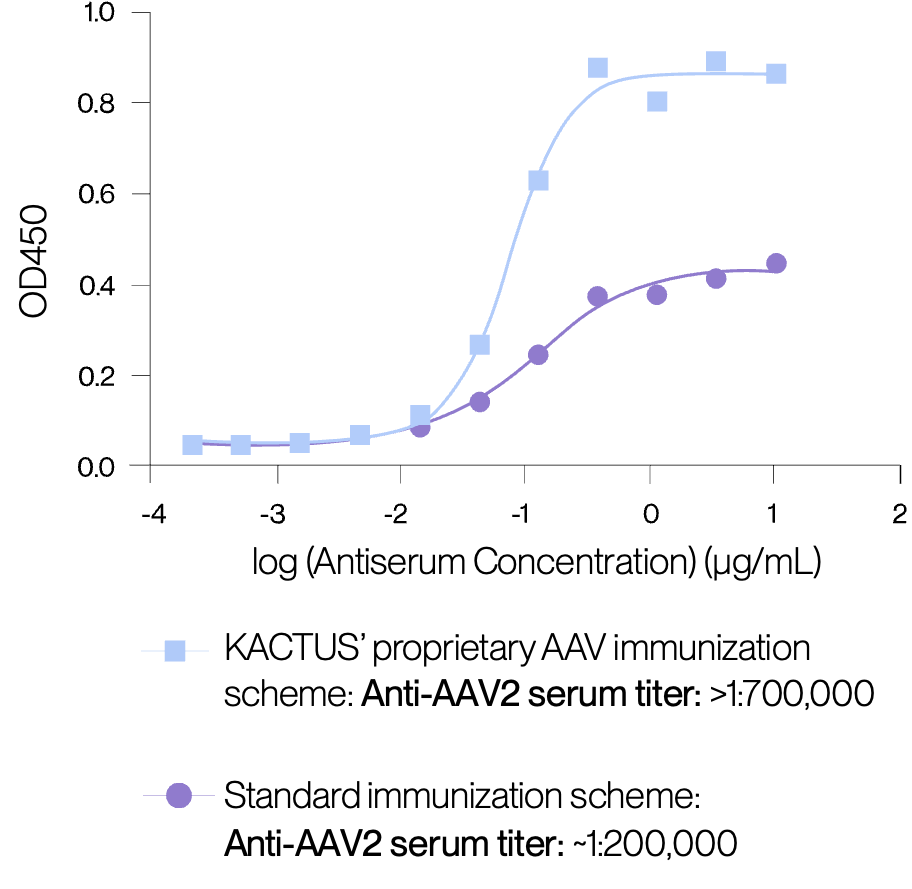
KACTUS’ AAV ELISA kits using recombinant monoclonal antibodies are developed using their proprietary immunisation scheme that overcomes the weak AAV immunogenicity. This method maintains a remarkable level of consistency as the serum titers within the same group of mice consistently align. Across all AAV serotypes, their technique consistently elevates AAV anti-serum titers, resulting in the production of high- performance monoclonal antibodies.
AAV2 Titration ELISA Kit Exhibits Near-Zero Cross-reactivity with AAV3
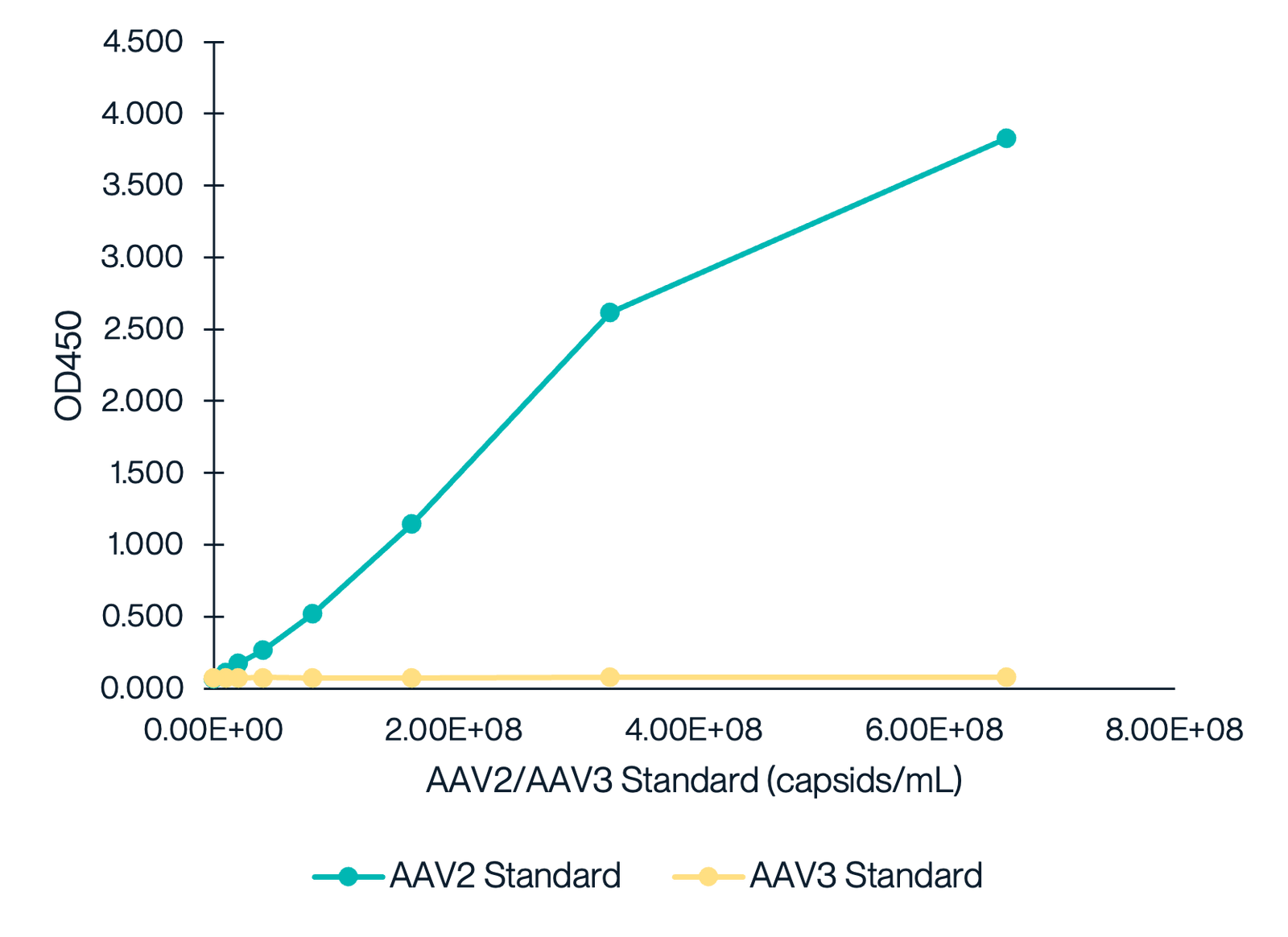
This graph demonstrates the cross-reactivity analysis of the AAV2 Titration ELISA Kit with AAV3. The data shows a dose-response curve for the AAV2 standard, while the AAV3 standard exhibits minimal reactivity, indicating the kit’s high specificity for AAV2 with no cross-reactivity detected for AAV3.
Overview of AAV ELISA Kits
Sandwich ELISA assays are a trusted technique for quantification of adeno-associated virus (AAV) capsid proteins, enabling researchers to determine viral titers with high specificity and sensitivity. KACTUS has developed a portfolio of AAV Titration ELISA kits that are designed to detect and quantify AAV capsid proteins across different AAV serotypes. These kits are broadly applicable in gene therapy research.
Due to the low immunogenicity of AAVs, and its growing role in gene therapy, they have developed a proprietary AAV immunisation scheme that produces high affinity anti-AAV monoclonal antibodies. They have extensively validated these ELISA assays to ensure that AAV titers can be accurately and consistently determined. KACTUS’ AAV ELISA assays can be applied to both preclinical and clinical research, supporting vector production, potency testing, and the development of novel therapeutics. These kits offer high accuracy, reproducibility, and specificity for adeno-associated virus detection.
How the AAV ELISA Kits Work
The assay uses a sandwich ELISA method to analyse samples with varying AAV concentrations. Their kits have a broad linear range to detect viral capsids over a dynamic range. AAV particles are captured using serotype-specific monoclonal antibodies immobilised on an ELISA plate. A biotinylated detection antibody is added that binds to the capsid proteins. Following the addition of TMB, a substrate reaction generates an absorbance signal, which is subsequently analysed against an 8-point standard curve to determine the concentration of viral particles.
This method is widely validated for research use, providing consistent and reproducible results for AAV vector characterisation and quality control. The specificity of the ELISA kit allows for differentiation between intact AAV particles and denatured capsid proteins. The antibodies are also highly serotype-specific, for reliable quantification of specific AAVs.
Product Advantages
Wide Linear Range
Batch Consistency
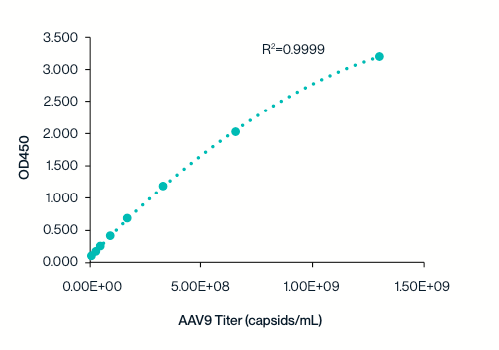
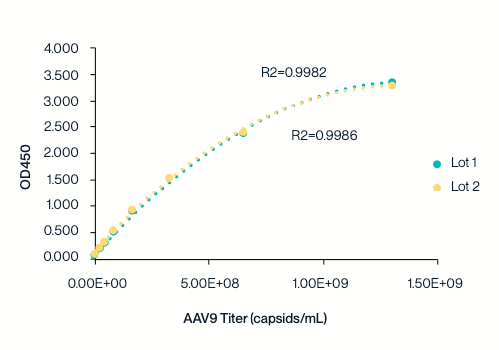
Example 8-point standard curve for the AAV9 Titration ELISA Kit. The quantitative range of this kit is 2.03×107 – 1.30109 capsids/mL.
KACTUS AAV9 Titration ELISA kit standard curve tested across two batches. The standard curve is highly reproducible and exhibits an R²>0.99.
Specificity to intact capsids
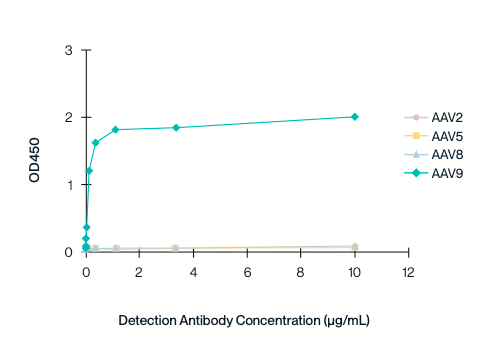
Specificity to individual serotypes
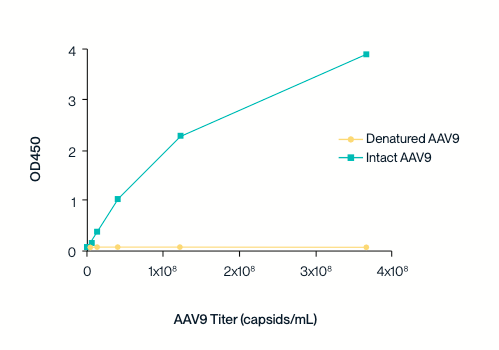
The ELISA sandwich assay was used to detect the binding of AAV9 antibodies to intact and denatured AAV9 capsid proteins. The AAV9 detection antibody does not exhibit binding to denatured AAV9 capsid protein.
The cross-binding of different concentrations of AAV9 antibodies to capsid proteins of other serotypes (AAV2/5/8/9) was detected by indirect ELISA. The AAV9 titration ELISA kit is highly specific to the AAV9 serotype and does not exhibit cross-reactivity with other AAV serotypes.
High Accuracy
High Precision
Sample | Runs | Average (capsids/mL) | Coefficient of Variation (%CV) |
|---|---|---|---|
| 6.5E+08 capsids/mL | n=10 | 6.18E+08 | 2.80% |
| 1.63E+08 capsids/mL | n=10 | 1.92E+08 | 1.90% |
| 2.03E+07 capsids/mL | n=10 | 2.17E+07 | 5.90% |
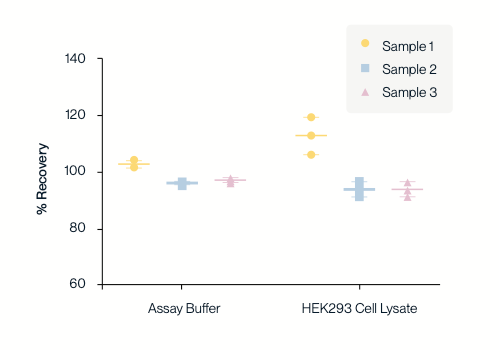
KACTUS AAV9 Titration ELISA kit was used to test the recovery rate of three AAV9 titers (high, medium, and low) in spiked dilution buffer (left) and HEK293 cell lysate (right). The sample recovery rates of AAV9 samples with different titers detected in the dilution buffer and cell lysate were all between 80% and 120%, indicating that this kit has high accuracy in detecting AAV9 titers.
The same batch of AAV9 test kits were used to test three different titer AAV9 samples (high, medium and low). Each sample was tested 10 times. The average and coefficient of variation (%CV) of these 10 titer tests was calculated. The %CV for all three sample concentrations (high, medium and low) was less than 10%, indicating that the AAV9 Titration ELISA kit has good intra-batch repeatability.
Caltag Medsystems is the distributor of KACTUS products in the UK and Ireland. If you have any questions about these products, please contact us.
