High-Quality Recombinant CD19 Proteins for B-Cell Malignancy Research and Antibody-Based Therapy Development
CD19 is a pivotal transmembrane glycoprotein involved in B cell activation and humoral immune responses, making it a critical focus for therapies targeting B-cell malignancies such as chronic lymphocytic leukaemia (CLL), acute lymphoblastic leukaemia (ALL), and various non-Hodgkin lymphomas. Its universal expression throughout B-cell development and pivotal role in activating signalling pathways make it an ideal focus for developing antibody-based treatments, including bispecific antibodies, ADCs, Fc-engineered antibodies, and CAR-T cell therapy.
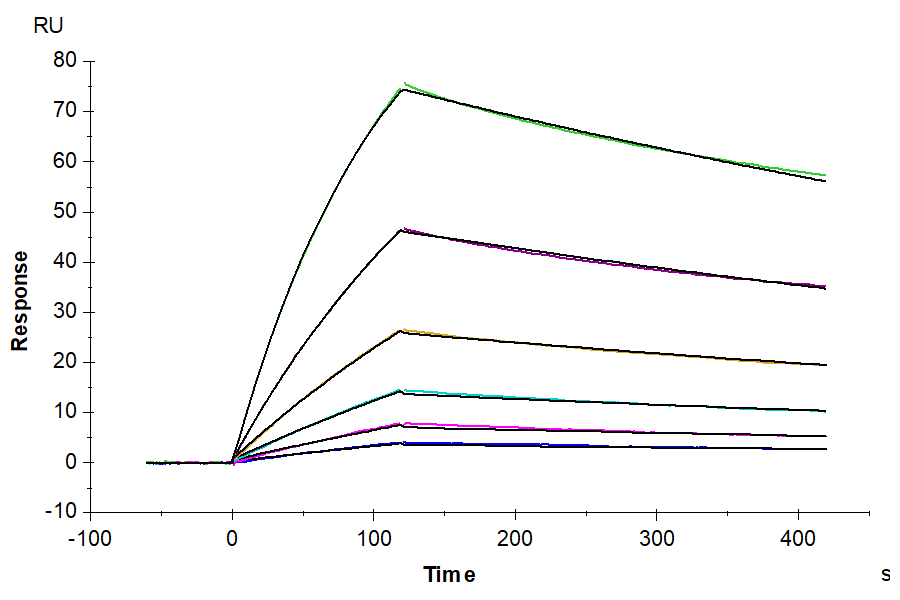
KACTUS’ high-quality CD19 proteins are designed for applications such as antibody screening, CAR-T cell therapy development and affinity measurements. Validated through ELISA, SPR and flow cytometry, these CD19 proteins ensure reliability and efficacy in your research.
Product Features
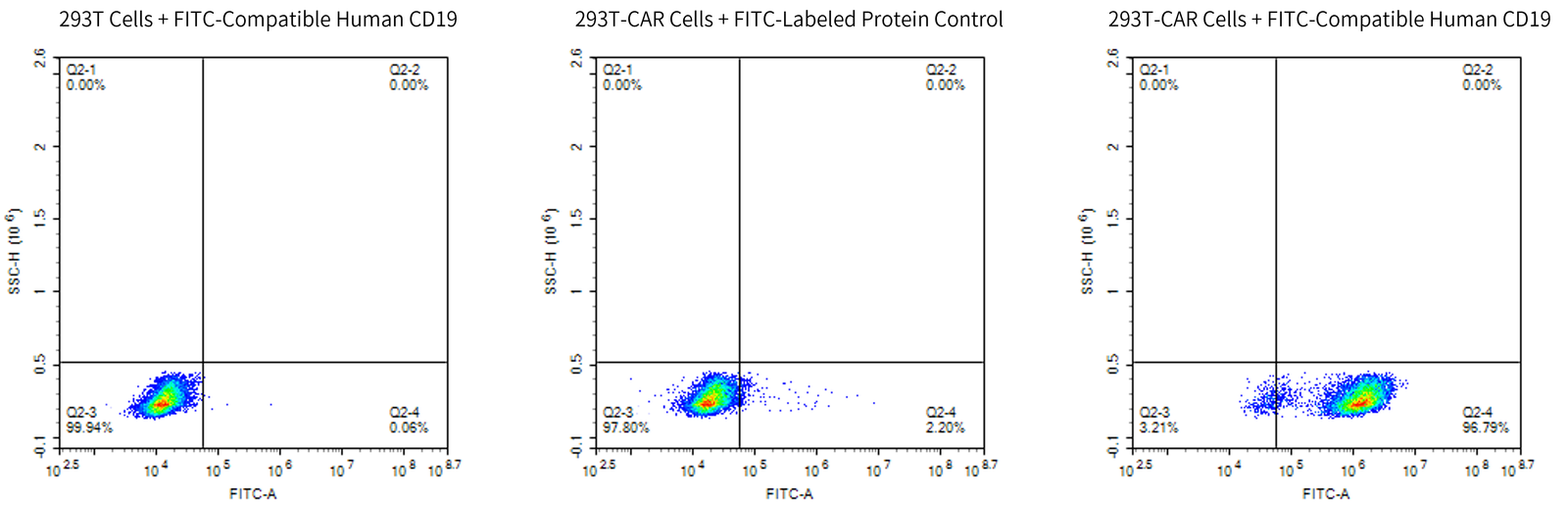
KACTUS’ recombinant CD19 protein products offer versatility and reliability, available in various species, including human, cynomolgus, rhesus macaque and mouse. These CD19 proteins have been validated for high binding affinity by SPR and ELISA. Their FITC-compatible human CD19 can be used for CAR detection via FACS. With off-the-shelf catalog products in stock, they ensure short lead times to meet your research needs efficiently.
- Various Species – Human, Cynomolgus, Rhesus Macaque, Mouse
- High Monomer Rate – Expressed with small tag (His) with optimal monomer rate
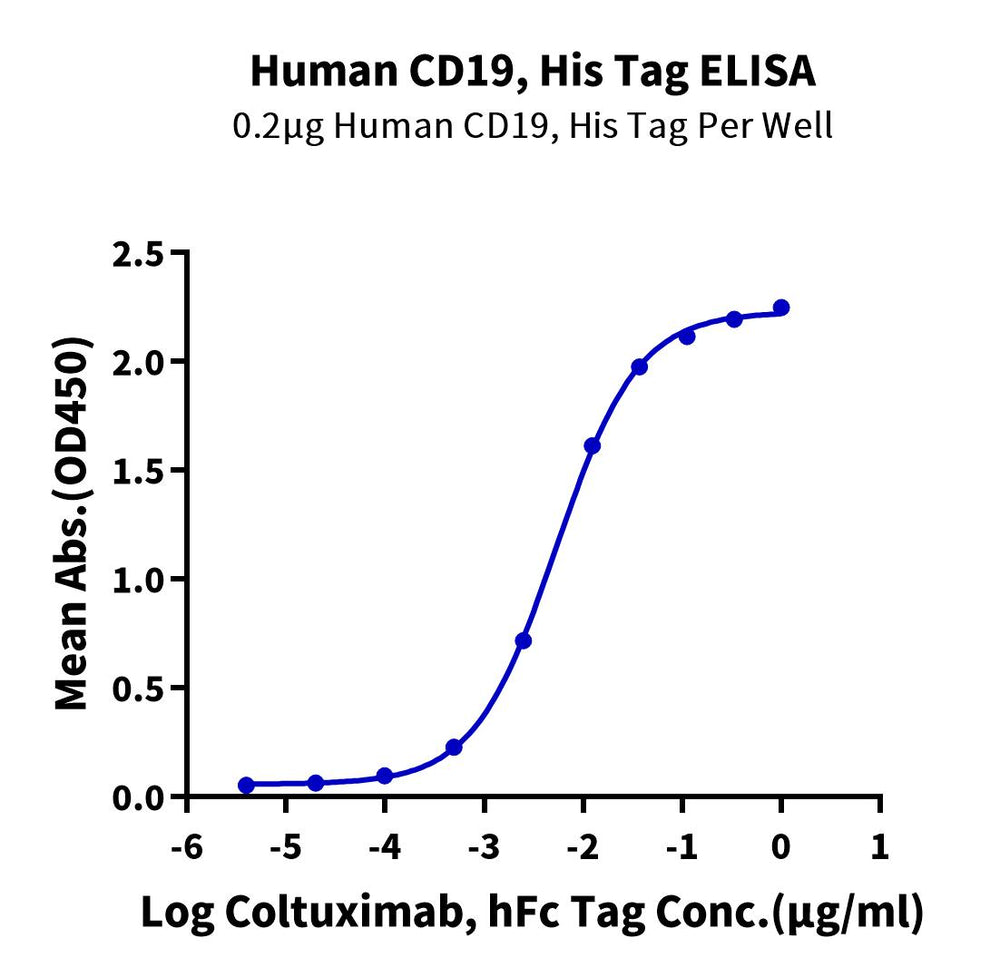
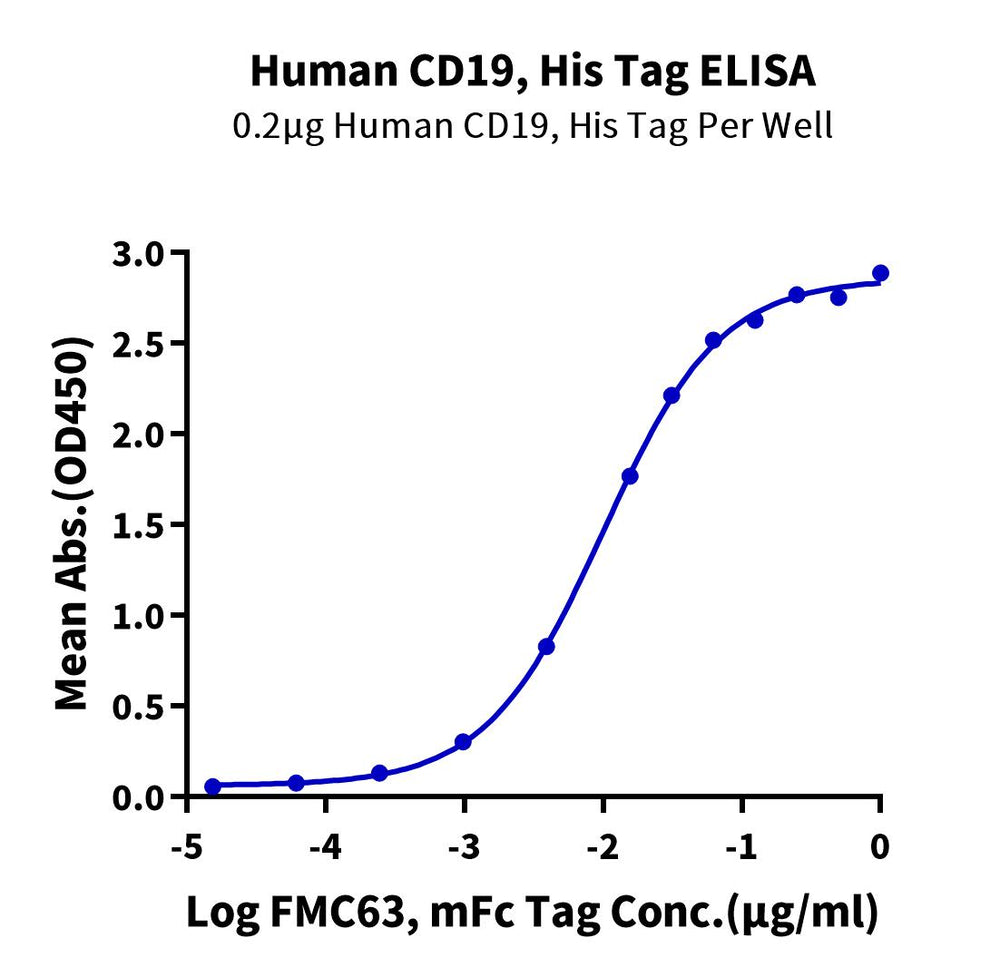
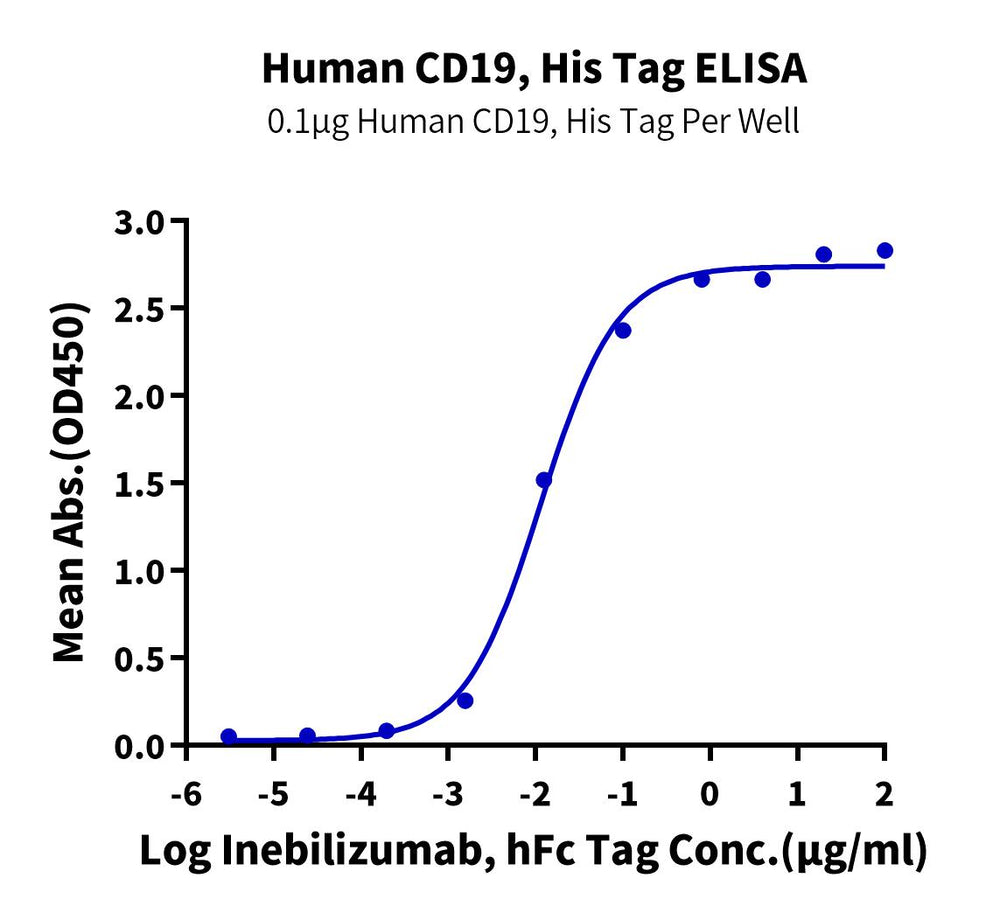
- High Binding Affinity – High binding affinity verified by SPR and ELISA
- CAR Detection – FACS Compatible Human CD19 for CAR Detection
- In Stock – Off-the-shelf catalog products with short lead time
Product Validation Data
KACTUS’ team has performed extensive binding affinity and activity verification of their CD19 protein products including FACS detection of anti-CD19 CAR cells as well as ELISA and SPR verification using anti-CD19 antibodies. Their CD19 products also undergo purity and identity testing using HPLC and Bis-Tris page.
Detection of Anti-CD19 CAR cells using FITC-compatible human CD19
High binding affinity verified by ELISA and SPR
Caltag Medsystems is the distributor of KACTUS products in the UK and Ireland. If you have any questions about these products, please contact us.
