Background
The key to linear mRNA therapy lies in efficiently synthesising high-quality mRNA in vitro and then delivering mRNA to the human body through a delivery system. The in vitro synthesis of mRNA cannot be done without high-quality raw material enzymes. KACTUS has a particular focus on the mRNA therapy market & have used their unique functional recombinant protein and high-activity enzyme production platform, SAMS™, to develop a full series of high-quality raw material enzymes required for in vitro synthesis of mRNA (GMP-Grade & GMP-Ready). Their GMP products have analytical method verification and undergo comprehensive quality release testing to meet the requirements for upstream raw material enzymes.
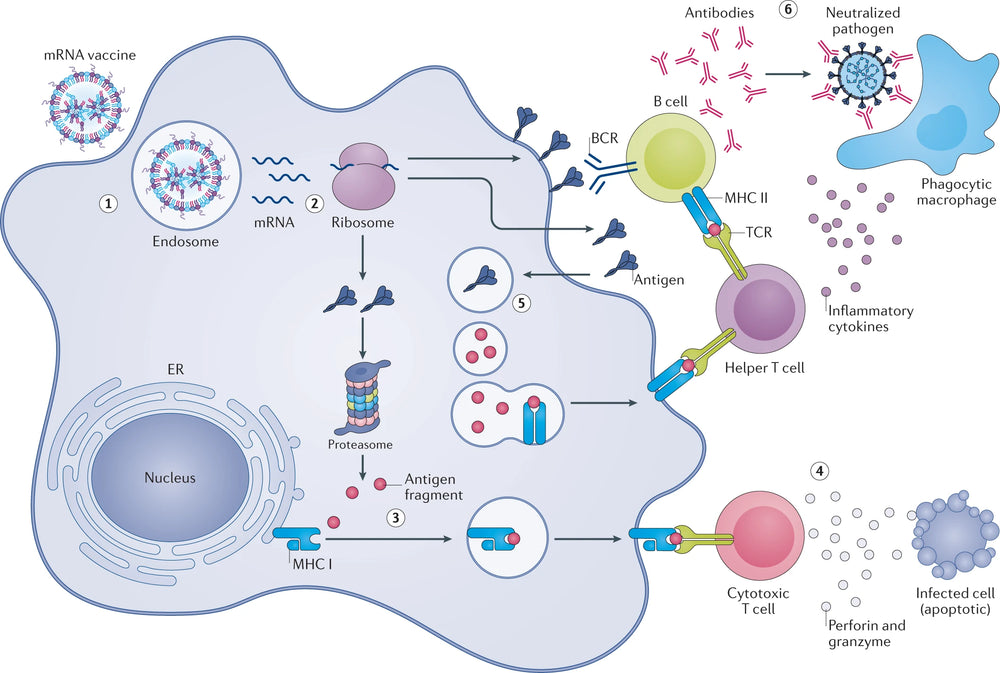
Enzymes for every step of RNA synthesis
Restriction Endonucleases for Plasmid Linearisation
The template for mRNA in vitro transcription is typically plasmid DNA, and needs to be linearised before transcription. Linearisation ensures the acquisition of mRNA transcripts of definite length and sequence. KACTUS provides restriction endonucleases such as BsaI, BspQI, SapI, XbaI, etc., for the preparation of linearised templates.
In Vitro Transcription (IVT) Reagents
In Vitro Transcription (IVT) is a technique that generates mRNA by mimicking the internal transcription process enzymatically, using linearised plasmid DNA as a template.
Available Products:
- MaxPure™ T7 RNA Polymerase, Research-Grade
- T7 RNA Polymerase, GMP-Grade
- Inorganic Pyrophosphatase, GMP-Grade
- Murine RNase Inhibitor, GMP-Grade
- DNase I, GMP-Grade
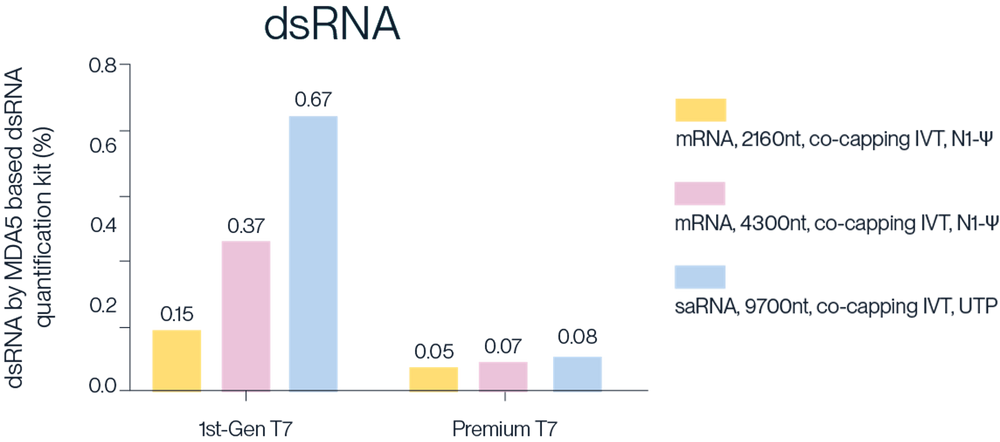
Note: MaxPure™ T7 RNA Polymerase is the commercial name for Premium T7 RNA Polymerase (RUO or GMP)
Enzymes for Capping & Tailing
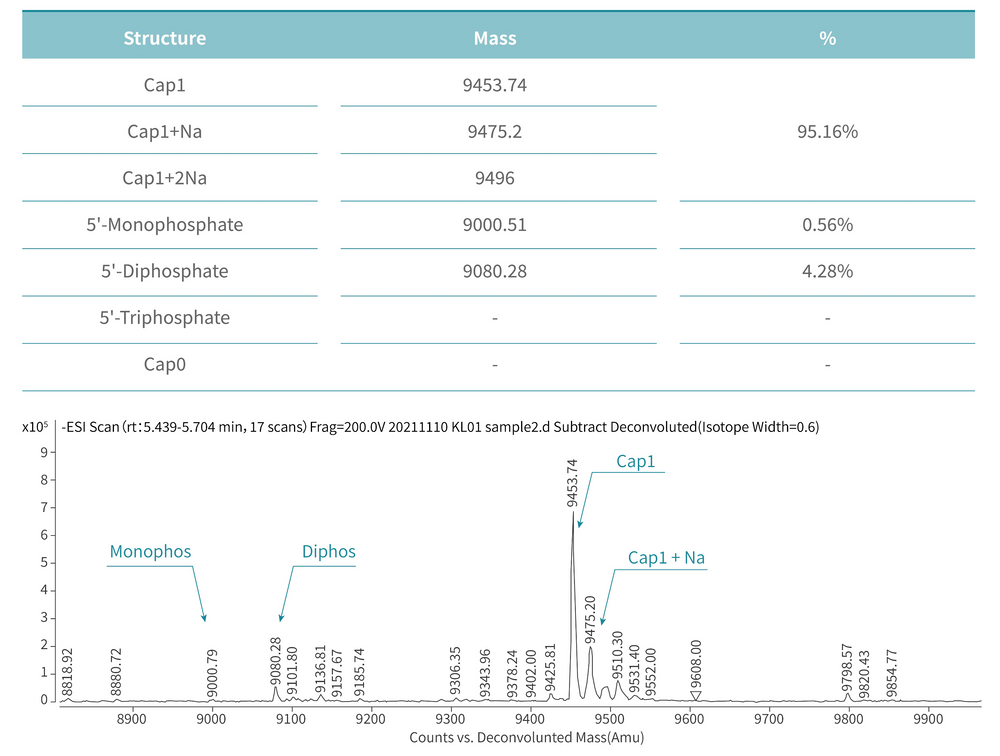
The mRNA produced by IVT has a triphosphate group at the 5’ end, which has strong immunogenicity, necessitating capping and tailing modifications in industrial production. KACTUS offers Vaccinia Capping Enzyme and mRNA Cap 2´-O-Methyltransferase needed for capping modifications, as well as E.coli Poly(A) Polymerase required for tailing modifications. To precisely quantify important indicators such as “capping rate” and “Poly(A) tail product length,” they have carefully established an LC-MS platform and developed mature mass spectrometry detection methods to control the release of enzyme products by batch. They can also provide capping rate and tailing determination services according to your needs.
Available Products:
- Vaccinia Capping Enzyme, GMP-Grade
- mRNA Cap 2´-O-Methyltransferase, GMP-Grade
- E.coli Poly(A) Polymerase
Enzymes for circRNA In Vitro Synthesis
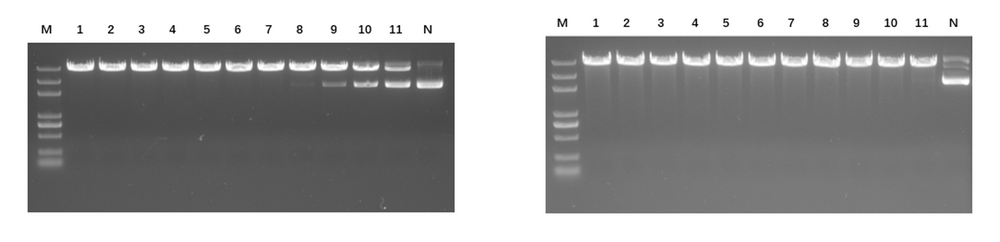
Linear mRNA obtained from IVT can be further circularised to obtain circRNA. The circularisation methods include Type I intron self-splicing, Type II intron self-splicing, T4 RNA Ligase, etc. KACTUS provides T4 RNA Ligase I, T4 RNA Ligase II, and RNase R for in vitro RNA circularisation and purification processing of circRNA.
Available Products:
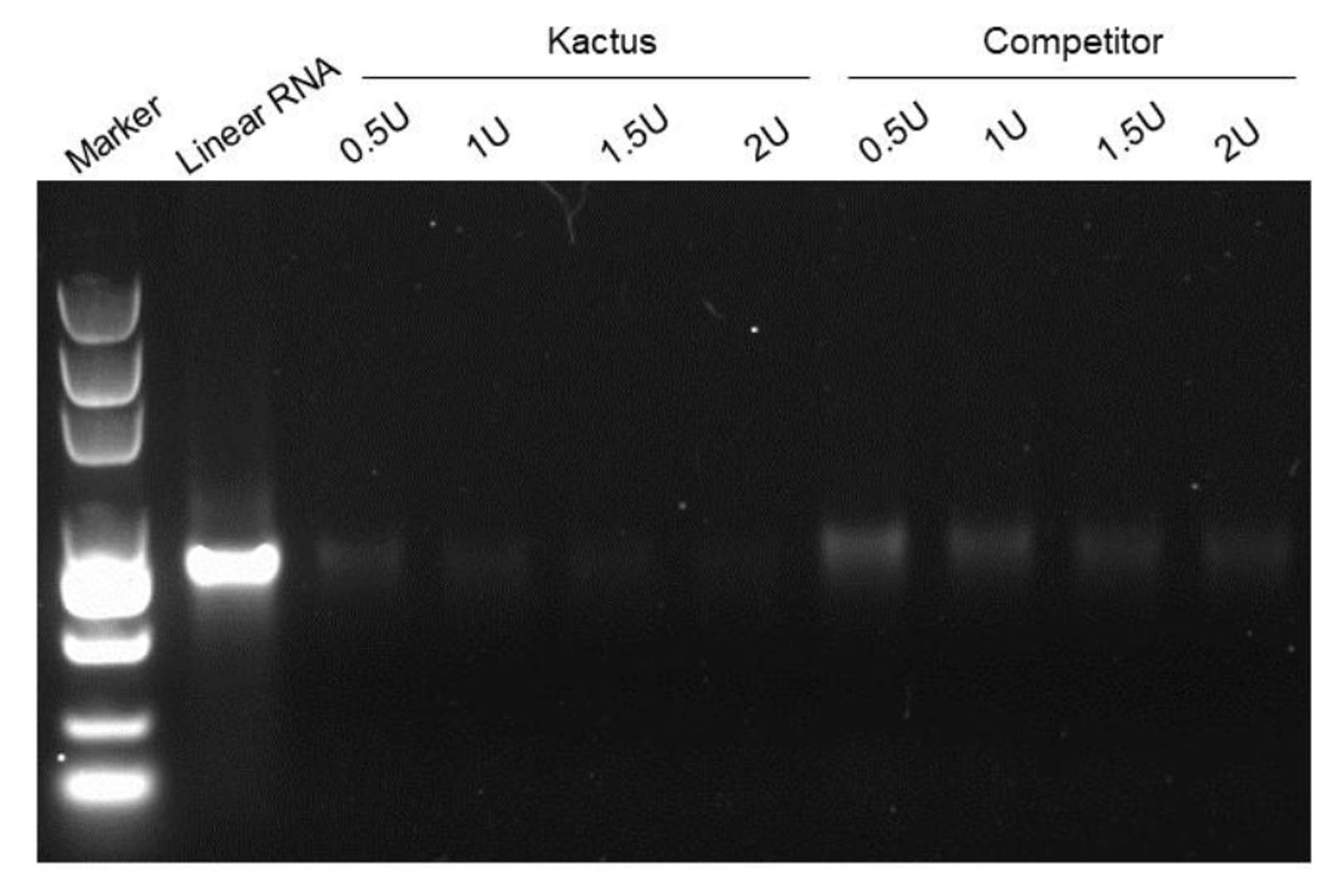
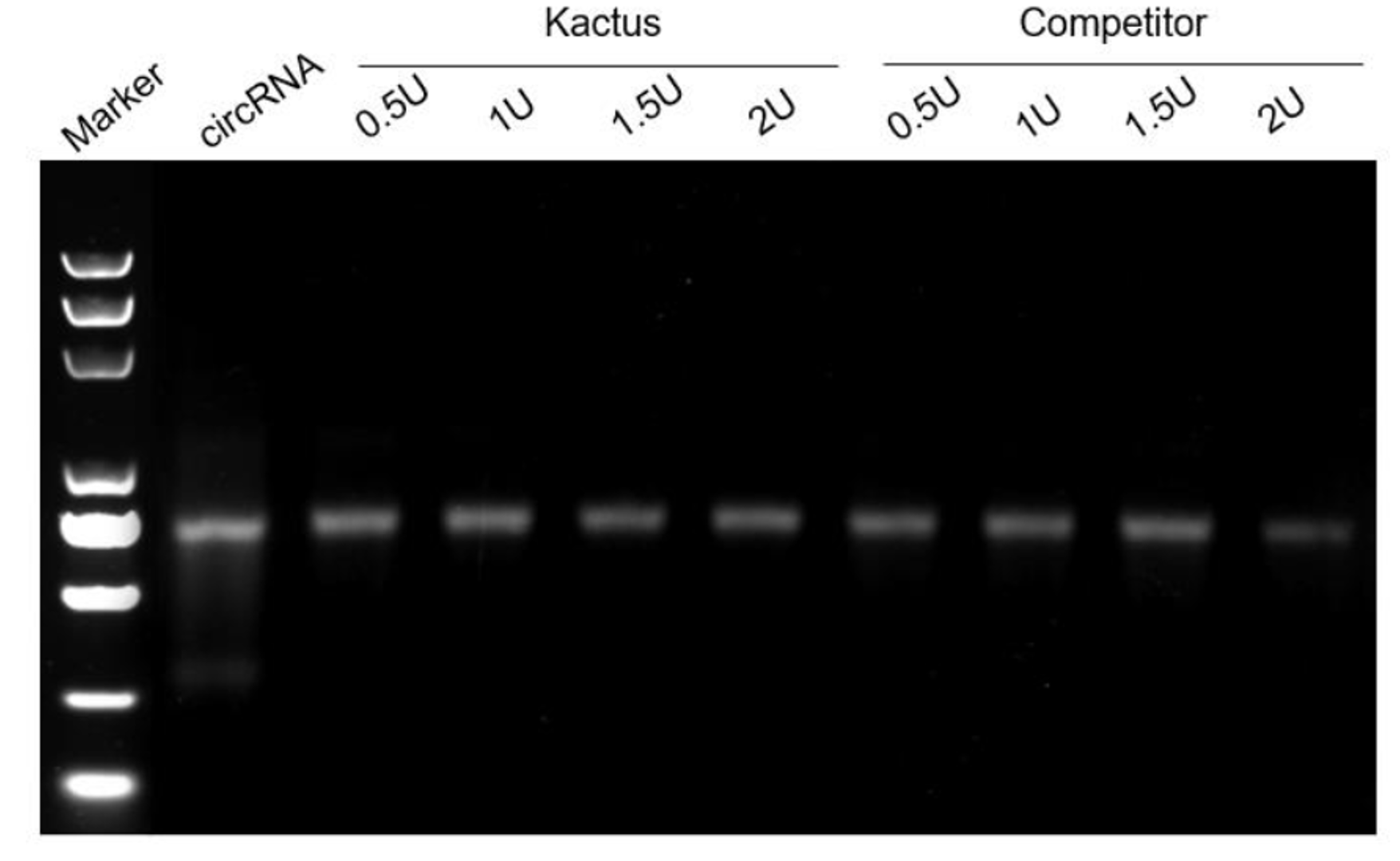
Figure 5. RNase R digests linear RNA (left) without affect circRNA (right).
Caltag Medsystems is the distributor of KACTUS products in the UK and Ireland. If you have any questions about these products, please contact us.
