Organoids are key 3-dimensional tissue structures and can be developed in many different types, they are used widely in drug discovery and various medical treatments due to their flexibility and how they can reflect cellular characteristics. When culturing organoids two growth factors are key to development, Wnt3a and FGF.
Afamin/Wnt3a
Why the Wnt signalling pathway is important
Wnt signalling is known to be involved in the early development, maintenance and regeneration of stem cells, and in cancer formation. Wnt signalling has also been found to play an important role in the growth and maintenance of these processes. In particular, Wnt3a has been revealed to be an essential niche component for maintaining the proliferation of Lgr5-positive stem cells in intestinal epithelial cells. It is used for the production of various digestive organoids such as the small intestine, large intestine, stomach, pancreas and liver. Although Wnt3a has been conventionally used for the culture of gut organoids, it is a fat-soluble protein, so it forms aggregates in serum-free medium and can not exert its activity sufficiently. In 2016, Mihara et al. found that high Wnt3a activity can be maintained by forming a complex with Wnt3a by Afamin, which is one of the components of serum. In addition, by using Afamin and Wnt3a complex for organoid culture, long-term culture of organoids becomes possible.
Mechanism of Wnt3a stabilisation by Afamin proteins
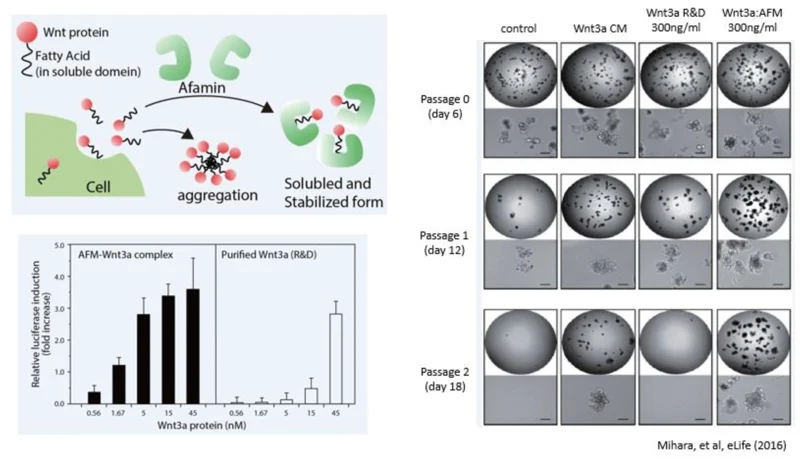
Due to Wnt3a proteins exhibiting strong hydrophobic properties, they are difficult to store and purify. This is the main issue for commercial Wnt3a products. MBL offers the solution to these issues with their stable and highly reactive Afamin/Wnt3a cell culture medium. When conjugated with Afamin, not only is the high activity maintained, but the ligand also does not aggregate in the aqueous solution.
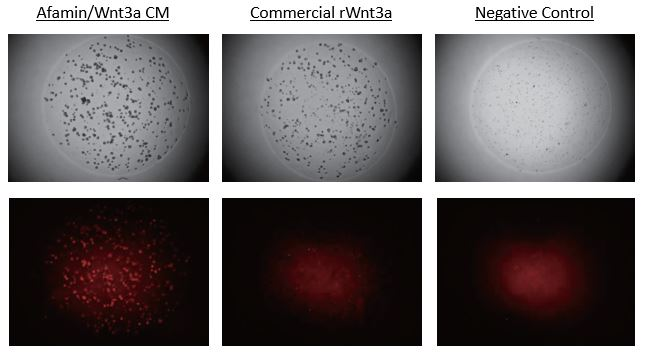
Afamin/Wnt3a CM increased Lgr5 Positive Stem Cells Expressed td Tomato
Images on the top panels show a bright field of human colour organoids. Images on the bottom panels show fluorescent LGR5 positive stem cells that express td tomato regulated by Lgr5 promoter. Afamin/Wnt3a CM maintained LGR5 positive stem cell growth is seen at greater levels compared with cell growth in commercial recombinant Wnt3a (300ng/mL).
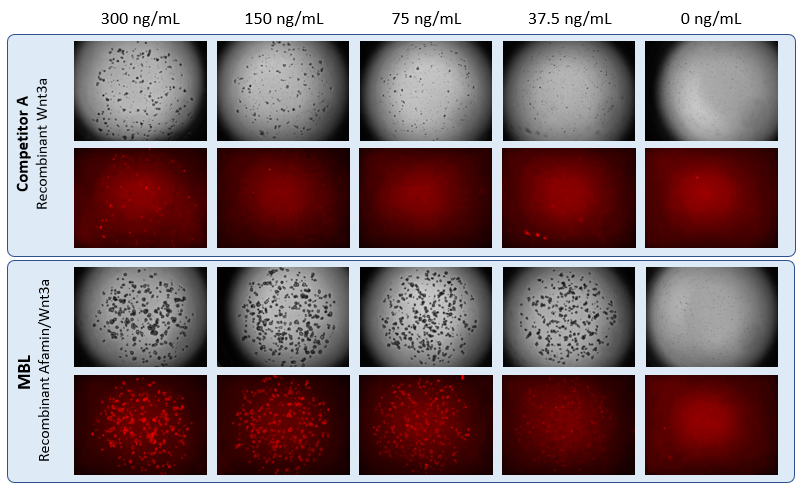
Recombinant Afamin/Wnt3a increased LGR5 Positive Stem Cells
An organoid strain expressing tdTomato regulated by Lgr5 promoter was cultured in the presence of Recombinant Afamin/Wnt3a and Recombinant Wnt3a of competitor A. It was confirmed that Recombinant Afamin/Wnt3a maintains LGR5-positive cells even at low concentrations.
MBL’s Afamin/Wnt3a Conditioned Medium vs Existing Wnt3a products
| MBL’s Afamin/Wnt3a Conditioned Medium | Recombinant Wnt3a | Wnt3a Conditioned Medium made in-house from Recombinant Wnt3a | |
| Wnt3a activity | High activity stabilised by Afamin protein | Insufficient activity for long-term organoid culture | High activity stabilised by serum |
| Quality/stability | Completed evaluation of Wnt signal activity sufficient for organoid culture | Wnt signal activity evaluation completed | Lot-to-lot variation causes large differences in activity evaluation |
| Presence or absence of serum? | Serum-free | Serum-free | Contains serum |
Afamin/Wnt3a is different from the wild-type proteins and using advanced techniques from Japan, has been designed to improve organoid culture. MBL has the exclusive commercial use license of Afamin/Wnt3a and is developing many new products to further simplify organoid culturing.
References
- K. Ikezawa, et al., Establishment of organoids using residual samples from saline flushes during endoscopic ultrasound-guided fine-needle aspiration in patients with pancreatic cancer., Endosc Int Open 10 (2022) [PMID: 35036290]
- K. Toshimitsu, et al., Organoid screening reveals epigenetic vulnerabilities in human colorectal cancer., Nat Chem Biol 18 (2022) [PMID: 35273398]
- Z. Wang, et al., Lactate promotes the growth of patient-derived organoids from hepatopancreatobiliary cancers via ENO1/HIF1α pathway and does not affect their drug sensitivities., Cell Death Discov 8 (2022) [PMID: 35443744]
- C. Cao, et al., Phenotypical screening on metastatic PRCC-TFE3 fusion translocation renal cell carcinoma organoids reveals potential therapeutic agents., Clin Transl Oncol (2022) [PMID: 35118587]
- K. Togasaki, et al., Wnt Signaling Shapes the Histologic Variation in Diffuse Gastric Cancer., Gastroenterology 160 (2021) [PMID: 33217450]
- S. Sugimoto, et al., An organoid-based organ-repurposing approach to treat short bowel syndrome., Nature 592 (2021) [PMID: 33627870]
- T. De Oliveira, et al., Effects of the Novel PFKFB3 Inhibitor KAN0438757 on Colorectal Cancer Cells and Its Systemic Toxicity Evaluation In Vivo., Cancers (Basel) 13 (2021) [PMID: 33671096]
- T. Nishina, et al., Interleukin-11-expressing fibroblasts have a unique gene signature correlated with poor prognosis of colorectal cancer., Nat Commun 12 (2021) [PMID: 33863879]
- T. Ebisudani, et al., Direct derivation of human alveolospheres for SARS-CoV-2 infection modeling and drug screening., Cell Rep 35 (2021) [PMID: 34038715]
- D. Kim, et al., 3D Organoid Culture From Adult Salivary Gland Tissues as an ex vivo Modeling of Salivary Gland Morphogenesis., Front Cell Dev Biol 9 (2021) [PMID: 34458260]
- EA. Farshadi, et al., Organoids Derived from Neoadjuvant FOLFIRINOX Patients Recapitulate Therapy Resistance in Pancreatic Ductal Adenocarcinoma., Clin Cancer Res 27 (2021) [PMID: 34580113]
- YW. Cho, et al., Patient-derived organoids as a preclinical platform for precision medicine in colorectal cancer., Mol Oncol (2021) [PMID: 34850547]
- S. Sugimoto, et al., Organoid Derivation and Orthotopic Xenotransplantation for Studying Human Intestinal Stem Cell Dynamics., Methods Mol Biol 2171 (2020) [PMID: 32705652]
- K. Nanki, et al., Somatic inflammatory gene mutations in human ulcerative colitis epithelium., Nature 577 (2020) [PMID: 31853059]
- N. Sasaki, et al., Development of a Scalable Coculture System for Gut Anaerobes and Human Colon Epithelium., Gastroenterology 159 (2020) [PMID: 32199883]
- S. Mae, et al., Expansion of Human iPSC-Derived Ureteric Bud Organoids with Repeated Branching Potential., Cell Reports 32 (2020) [PMID: 32726627]
- Y. Nanki, et al., Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing., Scientific Reports 28 (2020) [PMID: 32724113]
- K. Miyabayashi, et al., Intraductal transplantation models of human pancreatic ductal adenocarcinoma reveal progressive transition of molecular subtypes., Cancer Discov 10 (2020) [PMID: 32703770]
- K. Kawasaki, et al., Chromosome Engineering of Human Colon-Derived Organoids to Develop a Model of Traditional Serrated Adenoma., Gastroenterology 158 (2020) [PMID: 31622618]
- K. Kawasaki, et al., An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping., Cell 183 (2020) [PMID: 33159857]
- H. Oshima, et al., Stat3 is indispensable for damage-induced crypt regeneration but not for Wnt-driven intestinal tumorigenesis., FASEB J 33 (2019) [PMID: 30156908]
- K. Nanki, et al., Divergent routes toward Wnt and R-spondin niche independency during human gastric carcinogenesis., Cell 174 (2018) [PMID: 30096312]
- S. Sugimoto, et al., Reconstruction of the human colon epithelium in vivo., Cell Stem Cell 22 (2018) [PMID: 29290616]
- T. Seino, et al., Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression., Cell Stem Cell 22 (2018) [PMID: 29337182]
- H. Tiriac, et al., Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment., Gastrointest Endosc 87 (2018) [PMID: 29325707]
- H. Tiriac, et al., Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer., Cancer Discov 8 (2018) [PMID: 29853643]
- M. Fujii, et al., Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition., Cell Stem Cell 23 (2018) [PMID: 30526881]
- JS. Roe, et al., Enhancer Reprogramming Promotes Pancreatic Cancer Metastasis., Cell 170 (2017) [PMID: 28757253]
- E. Mihara, et al., Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/α-albumin., eLife 5 (2016) [PMID: 26902720]
Information provided by MBL.
Caltag Medsystems is the distributor of MBL products in the UK and Ireland. If you have any questions about these products, please contact us.
