During the 2016 State of the Union Address, President Obama called attention to a myriad of issues currently plaguing Americans. One of the many initiatives President Obama announced in response was a “moonshot” to cure cancer, referencing the flood of American technological innovation that led to the first man walking on the moon in 1969. Vice President Joe Biden was then handed the reins to the initiative, which has already been supported by Congress through the passing of a $264 million dollar funding increase to the National Cancer Institute.
of the Union Address, President Obama called attention to a myriad of issues currently plaguing Americans. One of the many initiatives President Obama announced in response was a “moonshot” to cure cancer, referencing the flood of American technological innovation that led to the first man walking on the moon in 1969. Vice President Joe Biden was then handed the reins to the initiative, which has already been supported by Congress through the passing of a $264 million dollar funding increase to the National Cancer Institute.
To do our part, ProSci would like to update you on how immunotherapy is being leveraged to battle cancer. Immunotherapy is the heart of multiple, promising cancer treatments in 2016. These treatments aim to harnesses the power of our highly sophisticated immune systems through immune checkpoint inhibitors, genetic modification of T cells, and manipulation of cytokine concentrations.
(View ProSci’s range of cancer products here)
Immune Checkpoint Inhibitors
The immune system communicates with the rest of the body through molecular mechanisms and signals, which certain cancers have mimicked or manipulated to shield themselves from eliciting an immune response. Immune checkpoint inhibitors aim to reprogram the immune system in order to activate an immune response despite contradictory molecular cues from cancer cells. These treatments are currently high risk due to the inability of the immune system to differentiate between healthy cells and cancer cells once immune checkpoints have been circumvented.
The two primary targets for immune checkpoint inhibitors are CTLA-4 and PD-1. CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) is a receptor that downregulates the immune system. By blocking the receptor with monoclonal antibodies, the immune system is effectively upregulated. To read about the PD-1 immune checkpoint, its ligands, and ProSci’s single domain PD-1 antibody, click here.
Example drugs: Ipilimumab
Radioimmunotherapy (RIT)Cancer Immunotherapy
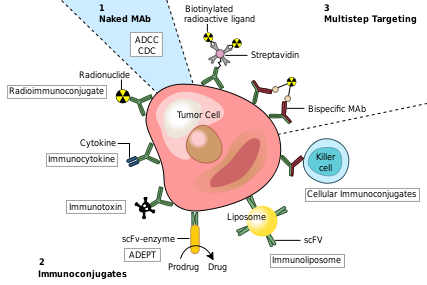 One of the key challenges in cancer treatments is to target and kill diseased cells without harming healthy cells in the process. Even the most effective potential treatments, such as immune checkpoint inhibitors, side effects are common and potentially lethal. RIT attempts to address this issue by pairing a monoclonal antibody with radioactive material, directing toxic substances to a specific subset of cells displaying a predetermined antigen. The antigen is often determined by harvesting the patient’s cancerous cells and identifying antigens unique to the malignancy. These antigens are then used to develop the appropriate monoclonal antibody, which is subsequently conjugated to a radioactivematerial.
One of the key challenges in cancer treatments is to target and kill diseased cells without harming healthy cells in the process. Even the most effective potential treatments, such as immune checkpoint inhibitors, side effects are common and potentially lethal. RIT attempts to address this issue by pairing a monoclonal antibody with radioactive material, directing toxic substances to a specific subset of cells displaying a predetermined antigen. The antigen is often determined by harvesting the patient’s cancerous cells and identifying antigens unique to the malignancy. These antigens are then used to develop the appropriate monoclonal antibody, which is subsequently conjugated to a radioactivematerial.
Example drugs: Ibritumomab tiuxetan
Antigen vaccines:
Antigens are used the same way in cancer research as they are in antibody development: to provoke an immune response. Cancer specific proteins or peptides can be used to stimulate an immune reaction to the malignant cells in a patient. Generally, antigen vaccines are not patient specific like other vaccines such as autologous tumor cells vaccines.
Cytokine Treatment:
Cytokines are key modulators of the immune system. They communicate with T lymphocytes and macrophages to active an immune response and can be used to artificially activate or increase an immune response in cancer patients. Cytokines are already being used as a cancer therapy known as interferon, but clinicians are learning how to utilize the innate molecules in new ways.
Interleukin-2 therapy has been a hot topic in cancer research and is administered through a variety of protocols. While some patients are administered IL-2 directly, others have immune cells found within the tumor (Tumor-infiltrating lymphocytes) treated with IL-2, multiplied, and then re-injected.
One danger implicit to cytokine treatments is a cytokine storm or hypercytokinemia: an uncontrolled, positive feedback loop between the cytokines and white blood cells which causes fever, swelling and nausea. Cytokine storms can be fatal.
Chimeric antigen receptor (CAR) T-cell therapy
CAR T-cell therapy has been hailed as one of the most effective new therapies in cancer research. In Chimeric antigen receptor T-cell therapy, T-cells are harvested from the patient and genetically modified to display chimeric antigen receptors, which bind to cancer cells. Once the cells are modified, they are infused into the patient. While the clinical trial results have been promising, the side effects are dangerous. There are a wide variety of cancer therapies that are currently in clinical trials, a large number of which can be categorized as immunotherapy. Many of these therapies involve antibodies and antibody conjugates, thanks to their specificity and relatively low risk side effects. ProSci is committed to developing the most reliable and effective antibodies against immune checkpoint inhibitors and given antigens as our personal initiative in the “Moonshot Against Cancer.”
Find out how ProSci can help your Antibody needs today!
References
http://www.cancer.org/treatment/treatmentsandsideeffects/treatmenttypes/immunotherapy/immunotherapy-whats-new-immuno-res
https://www.lls.org/treatment/types-of-treatment/immunotherapy
http://www.bloodjournal.org/content/119/12/2709?variant=long&sso-checked=true
Questions? Get in touch.
Originally posted by Prosci Inc.

