The Cleanascite™ reagent has been used extensively across a number of research applications to selectively removes lipids and their constituents (e.g cell debris, transgenic milk and egg yolk) for pretreatment of samples prior to purification. The reagent is a solid-phase, non-ionic adsorbent supplied as a suspension in saline, ready for use. Simply add, centrifuge and/or filter. The clarified supernatant is ready for subsequent downstream processing or analysis.
Cleanascite™: Key Features
- A high binding capacity for lipids with minimal cross-reactivity with proteins
- Effectively replaces chlorinated/fluorinated hydrocarbons (eg. freon) and it is environmentally friendly.
- Helps purify antibodies, recombinant proteins, nucleic acids, proteoglycans
- Ideal for clarifying ascites, serum, cell & tissue culture, bile and organ homogenates
- Clarifies saliva and faecal components
- Very low protein binding
- Does not bind to DNA, RNA, enzymes and proteins
- Leaves glycoproteins, antibodies, nucleic acids, hemoglobin, proteoglycans, nucleic acids, serum components(such as hormones, nutrients, globulins, clotting factors, transport proteins) alone
- Extends the life of membrane and chromatographic columns
- Enrichment of delipidated tissue samples
- Ideal for delipidation treatments for downstream processing of large-scale therapeutic proteins, enzymes and monoclonal antibodies.
Products Available
| Product Name | Size | Total Sample Volume That Can Be Processed* | Product Code |
| Cleanascite™ | 50 ml | 200 ml | X2555-50 |
| Cleanascite™ | 100 ml | 400 ml | X2555-100 |
| Cleanascite™ | 500 ml | 2000 ml | X2555-500 |
| Cleanascite™ | 1000 ml | 4000 ml | X2555-1000 |
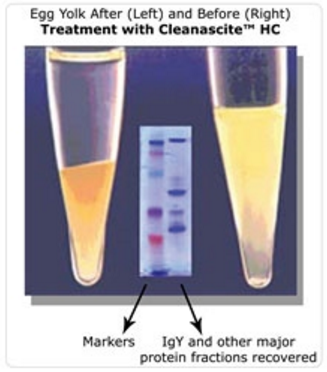
Featured Application – microRNA from Egg Yolk
Ben Wade, Michelle Cummins, Anthony Keyburn and Tamsyn M. Crowley. Isolation and detection of microRNA from the egg of chickens. BMC Research Notes 2016 9:283.
In brief, the article’s authors report a method for the reproducible and reliable isolation of miRNA from the albumen and yolk of chicken eggs. These methods will allow the investigation of epigenetic programming in chick development previously unknown, and how this impacts the nutritional value of eggs for human consumption. The article states “…400 µl aliquots of the yolk/lysis solution was dispensed into five 1.5 ml microcentrifuge tubes. To each of these aliquots 600 µl of Cleanascite™ was added followed by rigorous vortexing until the sample became homogenous. The Cleanascite™ removes the lipid from this high fat tissue that would otherwise interfere with the extraction process…”
“This is still another example of the versatility of Cleanascite™ as it now shown to help purify microRNA from egg yolk, a very lipid-rich tissue type. The exquisite selectivity profile of Cleanascite™ makes this possible.” states Swapan Roy, Ph.D., President and Founder of Biotech Support Group.
Featured Application – Vaccine Research
Vaccine research relies on the systemic immunogenic response to the vaccine candidate. To evaluate such a response, it is necessary to measure the antibodies from sera; a sample type with a diverse lipid profile between individuals. Because lipids can often impact antibody analysis by specific and non-specific matrix effects, for vaccine development, it is beneficial to deplete lipids prior to analysis. The Biotech Support Group product – Cleanascite™ has the necessary selectivity profile to support this demanding application. Read more here.
Caltag Medsystems are the exclusive UK distributor for Biotech Support Group – the manufactures of Cleanascite. For further information about their range. or for ordering enquiries please contact us.
References:
Serum
Taylor, Steven W., et al. “A high-throughput mass spectrometry assay to simultaneously measure intact insulin and C-peptide.” Clinica Chimica Acta (2016).
Bile
Lukic, Natalija, et al. ” An integrated approach for comparative proteomic analysis of human bile reveals overexpressed cancer-associated proteins in malignant biliary stenosis.” Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 1844.5 (2014): 1026-1033.
Danese, Elisa, et al. ” Assessment of bile and serum mucin5AC in cholangiocarcinoma: Diagnostic performance and biologic significance.”Surgery (2014).
Farina, Annarita, et al. ” Bile carcinoembryonic cell adhesion molecule 6 (CEAM6) as a biomarker of malignant biliary stenoses.” Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics (2013).
Boschetti, Egisto, and Pier Giorgio Righetti. Low-Abundance Proteome Discovery: State of the Art and Protocols. Newnes, 2013.
Wang W, Ai KX, Yuan Z, Huang XY, Zhang HZ. Different Expression of S100A8 in Malignant and Benign Gallbladder Diseases.Digestive diseases and sciences. 2012.
Hauser-Davis RA, Lima AA, Ziolli RL, Campos RC. First-time report of metalloproteinases in fish bile and their potential as bioindicators regarding environmental contamination. Aquatic Toxicology.2012;110-111:99-106
Farina A, Dumonceau JM, Frossard JL. Proteomic Analysis of Human Bile from Malignant Biliary Stenosis Induced by Pancreatic Cancer Journal of Proteome Research.2009; 8(1):159-69
Guerrier L, Claverol S, Finzi L et al. Contribution of solid-phase hexapeptide ligand libraries to the repertoire of human bile proteins. Journal of Chromatography.2007;1176(1-2):192-205
Chen Bo, Zheng Jian-wei, Wang Jian-ming, et al. Establishment and preliminary analysis of a 2-D human biliary map Chinese Journal of Hepatobiliary Surgery.2007
Chen B, Dong JQ, Chen YJ et al Two-dimensional electrophoresis for comparative proteomic analysis of human bile. Hepatobiliary & pancreatic diseases international.2007 Aug;6(4):402-6
Guerrier L, Claverol S, Finzi L et al Contribution of solid-phase hexapeptide ligand libraries to the repertoire of human bile proteins.Journal of Chromatography A.2007;1176(1-2):192-205
Kristiansen TZ, Bunkenborg J, Gronborg M et al A Proteomic Analysis of Human Bile Molecular and Cellular Proteomics.2004;3:715-728
Biological Matrices
Graeme T Clark, Paul J Russell, and Steven Westwood. Modification without impact: a case study in clinical assay failure due to lipemia. Bioanalysis; 2012: 4,(12):1421-1428
Egg Yolk
Ben Wade, Michelle Cummins, Anthony Keyburn and Tamsyn M. Crowley. Isolation and detection of microRNA from the egg of chickens. BMC Research Notes 2016 9:283.
Organ Homogenates
Myerson, J., He, L., Lanza, G., Tollefsen, D. and Wickline, S. Thrombin-inhibiting perfluorocarbon nanoparticles provide a novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. Journal of Thrombosis and Haemostasis.2011;9:1292-1300
Thakuria D, Schmidt O, Liliensiek AK. Field preservation and DNA extraction methods for intestinal microbial diversity analysis in earthworms. Journal of Microbiological Methods.2009;76(3):226-33
Cheng AM, Moore EE, Masuno T et al Normal Mesenteric Lymph Blunts the Pulmonary Inflammatory Response to Endotoxin. Journal of Surgical Research.2006;136(S2):166-171
McNally T, Mackie IJ, Machin SJ et al. Increased levels of beta 2 glycoprotein I antigen and beta 2 glycoprotein I binding antibodies are associated with a history of thromboembolic complications in patients with SLE and primary antiphospholipid syndrome British journal of rheumatology.1995 Nov;34(11):1031-6
Red Blood Cells
Antunes RF; Brandao C; Maia M; Arosa FA. Red blood cells release factors with growth and survival bioactivities for normal and leukemic T cells. Immunology and Cell Biology.2011;89(1):111-21
Tracheal Swab Samples
Li D, Wang J, Wang R, Li Y. A nanobeads amplified QCM immunosensor for the detection of avian influenza virus H5N1, Biosensors and Bioelectronics.2011;26(S10):4146-4154
Fu LM, Shinnick TM. Genome-wide exploration of the drug action of capreomycin on Mycobacterium tuberculosis using Affymetrix oligonucleotide GeneChips Journal of Infection.2007;54(S3):277-284
Fu LM, Shinnick TM. Genome-wide analysis of intergenic regions of mycobacterium tuberculosis H37Rv using affymetrix genechips. EURASIP journal on bioinformatics & systems biology.2007:23054
Tissue and Cell Culture
Alhamdani MS, Schroder C, Hoheisel JD. Analysis conditions for proteomic profiling of mammalian tissue and cell extracts with antibody microarrays. Proteomics.2010;10(17):3203-7
Czambel RK, Kharlamov A, Jones SC. Variations of brain endothelial nitric oxide synthase concentration in rat and mouse cortex.Nitric Oxide.2010;22(S1): 51-57
Plasma/Serum
McIntyre, John A., et al. ” Antiphospholipid autoantibodies as blood biomarkers for detection of early stage Alzheimer’s disease.” Autoimmunity0 (2015): 1-8.
Knight, Jason S., Wei Luo, Alexander A. O’Dell, Srilakshmi Yalavarthi, Wenpu Zhao, Venkataraman Subramanian, Chiao Guo et al. ” Peptidylarginine Deiminase Inhibition Reduces Vascular Damage and Modulates Innate Immune Responses in Murine Models of Atherosclerosis.” Circulation research (2014): CIRCRESAHA-113.
Palekar, Rohun U., et al. ” Thrombin-Targeted Liposomes Establish A Sustained Localized Anticlotting Barrier Against Acute Thrombosis.” Molecular pharmaceutics (2013).
Lijowski M, Caruthers S, Hu G. High-Resolution SPECT-CT/MR Molecular Imaging of Angiogenesis in the Vx2 Model Investigative Radiology.2009;44(1): 15–22
Turner JD, Langley RS, Johnston KL. Wolbachia Lipoprotein Stimulates Innate and Adaptive Immunity through Toll-like Receptors 2 and 6 to Induce Disease Manifestations of Filariasis The Journal of Biological Chemistry.2009;284:22364-22378
Torrelles JB, DesJardin LE, MacNeil J. et al Inactivation of Mycobacterium tuberculosis mannosyltransferase pimB reduces the cell wall lipoarabinomannan and lipomannan content and increases the rate of bacterial-induced human macrophage cell death Glycobiology.2009;19(7):743-755
Cho N, Chueh PJ, Kim C et al Monoclonal antibody to a cancer-specific and drug-responsive hydroquinone (NADH) oxidase from the sera of cancer patients. Cancer Immunology, Immunotherapy. 2002;51(3):121-9
Shapiro S, Beenhouwer DO, Feldmesser M et al. Immunoglobulin G Monoclonal Antibodies to Cryptococcus neoformans Protect Mice Deficient in Complement Component C3 Infect. Infection and immunity.2002;70(5):2598-604
Castro AR, Morrill We, Pope V. Lipid Removal from Human Serum Samples Clinical and diagnostic laboratory immunology.2000;7(2):197-199
Nussbaum G, Cleare W, Casadevall A et al Epitope Location in the Cryptococcus neoformans Capsule Is a Determinant of Antibody Efficacy The Journal of experimental medicine.1997;185:685-694
Nanoparticles
Human & Plasma Cell Lines
Pan, Dipanjan, et al. “A strategy for combating melanoma with oncogenic c-Myc inhibitors and targeted nanotherapy.” Nanomedicine 10.2 (2015): 241-251.
Plasma-Enzyme-Nanoparticle Mixture
Pan, Dipanjan, et al. “Anti-Angiogenesis Therapy in the Vx2 Rabbit Cancer Model with a Lipase-cleavable Sn 2 Taxane Phospholipid Prodrug using αvβ3-Targeted Theranostic Nanoparticles.” Theranostics 2014; 4(6):565-578
Microarray
Srinivasan, Harish.”Antibody microarray as a proteomic tool for effective diagnosis and prediction of prognosis in cancer.”(2014).
Saliva
Lucy E. DesJardin Isolation of M. tuberculosis RNA from Sputum Methods in Molecular Medicine.2001;48:133-139
Beenhouwer DO, Shapiro S, Feldmesser M et al. Both Th1 and Th2 Cytokines Affect the Ability of Monoclonal Antibodies To Protect Mice against Cryptococcus neoformans Infection and immunity.2001;69: 6445-6455
Desjardin LE, Perkins MD, Wolski K et al. Measurement of Sputum Mycobacterium tuberculosis Messenger RNA as a Surrogate for Response to Chemotherapy American journal of respiratory and critical care medicine.1999;160(1):203-10
Template Preparation Production Sequencing Protocols. Stanford Genome Technology Center
Vaccine Research (Cholesterol Removal From Human Serum)
Kamtchoua, Thierry, Monica Bologa, Robert Hopfer, David Neveu, Branda Hu, Xiaohua Sheng, Nicolas Corde, Catherine Pouzet, Gloria Zimmerman, and Sanjay Gurunathan. Safety and immunogenicity of the pneumococcal pneumolysin derivative PlyD1 in a single-antigen protein vaccine candidate in adults.Vaccine (2012).
Yeast assays
Lifang, et al. Balanced globin protein expression and heme biosynthesis improve production of human hemoglobin in Saccharomyces cerevisiae. Metabolic Engineering (2013).
