Basic biomedical research into many human diseases and disorders often starts with living, functional cells and allows extraordinary scientific and technological advances in medical science. Yet, potential delays in accessing these cells can hinder forward progress of research and postpone necessary treatments to patients.
Within the last few decades, basic biomedical research using living, functional human primary cells have translated into clinical applications. Clinical applications, such as cell therapy, is a way to treat and cure diseases and disorders.
Primary cells are a critical raw material used in basic biomedical research and clinical applications and, therefore, when incorporating living cells into a study, it is essential they are of high-quality, as the cellular raw material is fundamental to get precise and relevant data for any study.
Time can be as critical as the outcome of your experiment.
For human primary cells, fresh cells are the ideal raw material as fresh cells maintain high viability and quality within the first 24hrs of collection from a donor. But as time persists the quality of the product declines.
Delays in receiving or using your cellular raw material can compromise downstream experiments and postpone the collection of data, which can set back a novel publication or the launch of a new product. These delays can likewise incur costs associated with personnel time, use of specific facilities (i.e., flow cytometry facilities), and reagents and equipment.
Now imagine a scenario in which there was a way to preserve the quality of the cellular raw material until you were ready to use it?
To avoid delays, StemExpress now provides Frozen Leukopaks®. These Leukopaks® offer convenience and flexibility of TIME for the researcher.
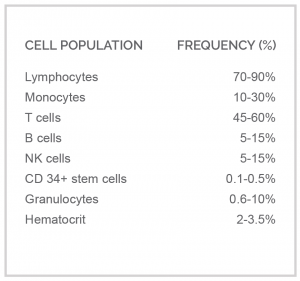
Like our Fresh Leukopak®, the new Frozen Leukopak® is a leukapheresis product from a single donor that provides an enriched peripheral blood mononuclear cell (PBMC) fraction composed of lymphocytes and monocytes with low granulocytes and red blood cells (Figure 1).
Figure 1. Average cell frequencies gathered from Leukopaks® before cryopreservation.
Within hours of collection from a donor at our FDA-registered Stem Cell Collection Center, the Leukopak® is frozen in cryopreservation media using a controlled rate freezer. The freezing of the Leukopak® right after collection maintains the viability of the cells for short-term storage and transportation.
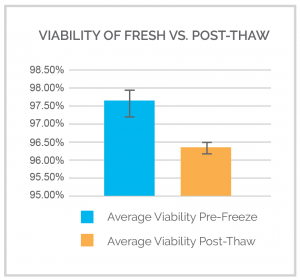
In a comparison study, we analyzed the viability of the same Leukopak® at the time of collection and post-thaw. Using our Leukopak® Thawing Protocol, we were able to show average cell viability of 96.33% post-thaw as compared to 97.56% for a freshly collected Leukopak® (Figure 2). Cell recoveries were >90%. It is important to follow our established Leukopak® Thawing Protocol to get the best viability and recovery of your cells.
Figure 2. Viability comparison of fresh and thawed Leukopaks®. Average viability of fresh Leukopaks taken at the time of collection was 97.56% while the average post-thaw viability taken 7 days after cryopreservation was 96.33%.