Suramin is over 100 years old and is still used to treat the first stage of acute human sleeping sickness, caused by Trypanosoma brucei rhodesiense. Suramin is a multifunctional molecule with a wide array of potential applications, from parasitic and viral diseases to cancer, snakebite and autism.
When suramin was introduced for the treatment of African sleeping sickness in 1922, it was the first urea-based drug to be approved in clinics and one of the first anti-infective agents that had been developed in a medicinal chemistry program. The development of suramin was a breakthrough in the emerging field of chemotherapy. Starting from the anti-trypanosomal activity of the dye trypan blue, synthesised in 1904 by Paul Ehrlich, Bayer made a series of colourless and more potent derivatives. To avoid skin staining as a side effect, and therefore looking for colourless derivatives, Oskar Dressel, Richard Kothe, and Bernhard Heymann at Bayer replaced the azo moieties present in trypan dyes with amido and ureyl linkers. These structural modifications preserved the conjugation between the aromatic rings but did not confer a peculiar colouration to the molecule. All urea derivatives showed higher anti-trypanosomal activities compared to the original dyes. Particularly, suramin (Molecule 205) was the best-in-class compound and it entered into therapy for the treatment of Human African Trypanosomiasis (HAT).
Despite its poor bioavailability and its intrinsic toxicity, through the years suramin was still widely used to treat HAT, and its use has been extended to other pathological conditions, including other parasitic infections (such as leishmaniasis and malaria) and viral infections (such as HIV, Chikungunya, Ebola, Dengue, Rift Valley fever, Zika or SARS-CoV2), although with limited effects. The antiviral and anti-bacteriophage activities of suramin have been known for a long time. It was found to inhibit retroviral reverse transcriptase, which served as a rationale to test suramin against human immunodeficiency virus (HIV). For other viruses it was postulated to inhibit viral entry to the host cells, inhibiting RNA synthesis or interfering with early steps of the replication cycle.
In addition, Suramin has also been evaluated as an anticancer agent against diverse neoplastic diseases such as melanoma, breast, prostate, bladder, brain, and lung tumours. None of these trials proved the efficacy of suramin as an anticancer agent in monotherapy. Conversely, suramin was shown to be an effective chemosensitiser in in vivo models by enhancing the efficacy of other anticancer drugs such as cyclophosphamide, adriamycin, mitomycin C, taxol and carboplatin in mice.
Suramin has also been described as a protective agent (antidote) in several studies, which have been attributed to basically 3 biological activities; i) the inhibition of thrombin (and thrombin-like proteases of snake venom), ii) the inhibition of phospholipase A2 (common constituents of metazoan venoms), and iii) the inhibition of purinergic signalling. Suramin’s ability to block P2 purinergic, G protein-coupled receptors may counteract the action of neurotoxins that trigger arachidonic acid signalling. A possible explanation is that suramin prevents the activation of ATP receptors at the motor nerve ending, which otherwise would depress Ca2+ currents and reduce acetylcholine release at the presynaptic membrane. Suramin also inhibits connexin channels of the tight junction, thereby suppressing ATP release and protecting cells from pore-forming bacterial toxins, such as hemolysin.
Over the last years, further potential applications of suramin have been described, such as a scaffold for medicinal chemistry, as an adjuvant for vaccination, used as an additive in CHO production media, or for the treatment of autism spectrum disorders (ASD).
Most recently, suramin has been shown to be an effective inhibitor of dsDNA-induced inflammation. Suramin has been shown to inhibit several dsDNA-binding proteins, including cGAS (inhibiting cGAS-STING and TLR9 mediated inflammatory responses), Mcm1040 and DNA topoisomerase II, and most recently also the AIM2 inflammasome, probably blocking DNA binding sites by mimicking the DNA sugar-phosphate backbone due to their extended, polysulfated anionic structures. Suramin inhibited AIM2-dependent ASC oligomerisation, caspase-1 and IL-1β processing and is a clinical drug that reversibly inhibits the AIM2 inflammasome in humans and mice.
Literature References:
- Suramin – a potent reversible and competitive inhibitor of complement systems: J.S. Fong & R.A. Good; Clin. Exp. Immunol. 10, 127 (1972)
- Suramin: a potent ATPase inhibitor which acts on the inside surface of the sodium pump: P.A. Fortes, et al.; Biochim. Biophys. Acta 318, 262 (1973)
- Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses: E. De Clerq; Cancer Lett. 8, 9 (1979)
- Differential inhibition of various deoxyribonucleic and ribonucleic acid polymerases by suramin: K. Ono, et al.; Eur. J. Biochem. 172, 349 (1988)
- Suramin, an anti-cancer drug, inhibits protein kinase C and induces differentiation in neuroblastoma cell clone NB2A: C.E. Hensey, et al.; FEBS Lett. 258, 156 (1989)
- Suramin analogues as subtype-selective G protein inhibitors: M. Freissmuth, et al.; Mol. Pharmacol. 49, 602 (1996)
- Suramin is an active site-directed, reversible, and tight-binding inhibitor of protein-tyrosine phosphatases: Y.L. Zhang, et al.; J. Biol. Chem. 273, 12281 (1998)
- Suramin suppresses growth, alkaline-phosphatase and telomerase activity of human osteosarcoma cells in vitro: K. Trieb & H. Blahovec; Int. J. Biochem. Cell Biol. 35, 1066 (2003)
- Suramin interaction with human alpha-thrombin: inhibitory effects and binding studies: R.Q. Monteiro, et al.; Int. J. Biochem. Cell Biol. 36, 2077 (2004)
- Functional regulation of the cardiac ryanodine receptor by suramin and calmodulin involves multiple binding sites: A.P. Hill, et al; Mol. Pharmacol. 65, 1258 (2004)
- Structure-activity studies on suramin analogues as inhibitors of NAD+-dependent histone deacetylases (sirtuins): J. Trapp, et al.; ChemMedChem 2, 1419 (2007)
- Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin: A. Schuetz, et al.; Structure 15, 377 (2007)
- Discovery and mechanistic study of a class of protein arginine methylation inhibitors: Y. Feng, et al.; J. Med. Chem. 53, 6028 (2010)
- The trypanocidal drug suramin and other trypan blue mimetics are inhibitors of pyruvate kinases and bind to the adenosine site: H.P. Morgan, et al.; J. Biol. Chem. 286, 31232 (2011)
- Neutralization of Apis mellifera bee venom activities by suramin: C.Z. El-Kik, et al.; Toxicon 67, 55 (2013)
- Suramin inhibits helicase activity of NS3 protein of dengue virus in a fluorescence-based high throughput assay format: C. Basavannacharya & S.G. Vasudevan; BBRC 453, 539 (2014)
- Suramin inhibits cullin-RING E3 ubiquitin ligases: K. Wu, et al.; PNAS 113, E2011 (2016)
- Suramin is a potent inhibitor of Chikungunya and Ebola virus cell entry: L. Henss, et al.; Virol. J. 13, 149 (2016)
- Suramin is a novel competitive antagonist selective to α1β2γ2 GABAA over ρ1 GABAC receptors: H. Luo, et al.; Neuropharmacol. 141, 148 (2018)
- Suramin potently inhibits cGAMP synthase, cGAS, in THP1 cells to modulate IFN-β levels: M. Wang, et al.; Future Med. Chem. 10, 1301 (2018)
- The anti-parasitic agent suramin and several of its analogues are inhibitors of the DNA binding protein Mcm10: C.N. Paulson, et al.; Open Biol. 9, 190117 (2019)
- Evaluating the impact of suramin additive on CHO cells producing Fc-fusion protein: J.-H. Lim, et al.; Biotechnol. Lett. 41, 1255 (2019)
- Suramin inhibits SARS-CoV-2 infection in cell culture by interfering 2 with early steps of the replication cycle: C. Salgado, et al.; Antimicrob. Agents Chemother. 64, e00900 (2020)
- 100 Years of Suramin: N. Wiedemar, et al.; Antimicr. Agents Chemother. 64, e01168-19 (2020) (Review)
- High Throughput Screening Targeting the Dengue NS3-NS5 Interface Identifies Antivirals against Dengue, Zika and West Nile Viruses: S.N.Y. Yang, et al.; Cells 11, 730 (2022)
- Discovery of an inhibitor of DNA-driven inflammation that preferentially targets the AIM2 inflammasome: J.P. Green, et al.; iScience 26, 106758 (2023)
Suramin . sodium salt – A Multifunctional Research Reagent
AG-CR1-3575 (50 mg & 250 mg sizes from stock)
AG-CR1-3575V (50 mg, 250 mg and 1 g sizes & Bulk from stock)
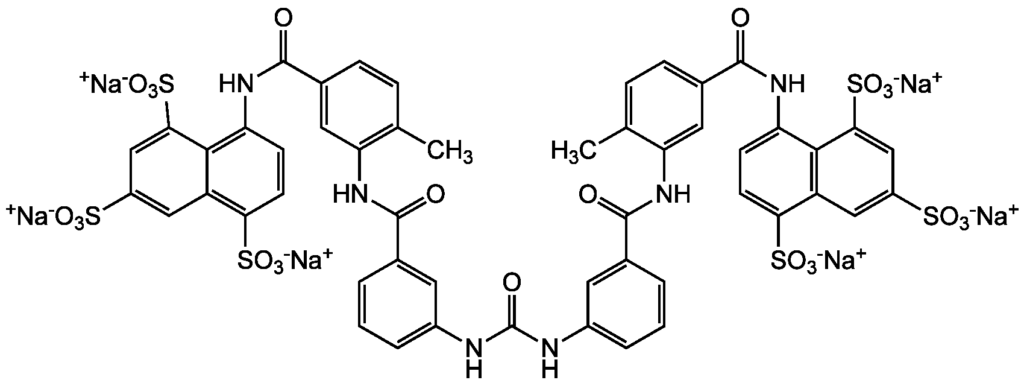
Suramin is a large molecule that carries six negative charges at physiological pH that binds to and therefore inhibits various proteins. Indeed, a large number of enzymes have been shown to be inhibited by suramin. Suramin inhibits many glycolytic enzymes, it decreases the activities of a large number of enzymes involved in DNA and RNA synthesis and modification: DNA polymerases, RNA polymerases, reverse transcriptase, telomerase, and enzymes involved in winding/unwinding of DNA. Suramin inhibits histone- and chromatin-modifying enzymes like methyltransferases and sirtuin histone deacetylases. Suramin is also an inhibitor of other sirtuins and protein kinases, phospholipase A2, protein tyrosine phosphatases, and different serine and cysteine proteases. Suramin further inhibits the Na+, K+-ATPase and other ATPases, certain classes of GABA receptors, and several G protein-coupled receptors. Suramin showed inhibitory effects against components of the coagulation cascade and the complement system and against deubiquitinating enzymes. Besides its many inhibitory activities, suramin activates certain nuclear receptors that act as transcription factors and intracellular calcium channels.
AdipoGen Life Sciences is an original Manufacturer of Suramin. Available from stock.
Product Specifications:
CAS: 129-46-4
Source: Synthetic
Purity: >98% Pharmaceutical Grade (#AG-CR1-3575)
>98% HPLC (#AG-CR1-3575V)
Identity: Determined by 1H-NMR
Solvents: Soluble in water (>50mM) or DMSO (>10mM).
Water Content: <15%
Sodium Content: 8 – 9.5%
For Research Use Only (RUO)
Contact us to inquire about BULK Pricing!
Originally posted on adipogen.com/suramin
Caltag Medsystems is the distributor of Adipogen products in the UK and Ireland. If you have any questions about these products, please contact us.
