Understanding Leukapheresis
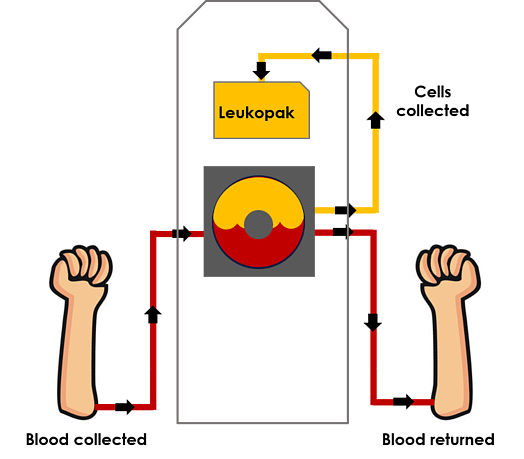
Leukapheresis is derived from the Latin words “leuk,” meaning white, and “aphaeresis,” meaning to take away. Put together, leukapheresis describes the collection of leukocytes — also known as white blood cells or immune cells — using a Spectra Optia® system. A donor reclines in a chair and blood is drawn through a vein in one arm. An apheresis machine removes white blood cells from the superfluous biofluids, separates them based on density and collects them in a collection bag known as a leukopak. The remaining red blood cells and platelets are then returned to the same donor through a vein in their other arm. (The white blood cells that have been removed will be replaced quickly.) The white blood cells collected are frequently referred to as peripheral blood mononuclear cells (PBMCs), and contain B cells, T cells, stem/progenitor cells and dendritic cells. Leukapheresis allows for a concentrated, pure method to obtain PBMCs from a single individual donor.
Leukopaks are used when a large number of PBMCs (Table 1) are needed from an individual donor, such as drug screening (immuno-oncology and non-oncology), cell therapy (research and development) and gene therapy. Sourcing healthy donor PBMCs allows for the use of single donor cell populations with greater viability and less inter- and intra-assay variability, as well as to develop well-characterized methods of production for autologous therapies (cell therapies where a patient’s own cells are modified for treatment). For these cell therapy developments, large numbers of PBMCs sourced from a single donor are important for creating relevant biological assays, as well as for quality control testing. In these cases, healthy donors are matched to patients in terms of demographic criteria, such as age and gender, and physiological criteria like HLA type, which can provide valuable insights in the progress of the patients’ own autologous cell therapy.
Table 1. Total Cell Volume per Leukopak size
| ¼ leukopak | 1-2 Billion Cells |
|---|---|
| ½ leukopak | 3-4 Billion Cells |
| Full leukopak | 6-8 Billion Cells |
Table 2. Average percentage cell population in standard and mobilized Leukopak.
| Leukopak | G-CSF | Mozobil | |
|---|---|---|---|
| T Cells | 57%, range 45-60% | ||
| CD3 + T Cells | Increased percentage than G-CSF | ||
| CD4+ T Cells | Increased percentage than G-CSF | ||
| CD8+ T Cells | Increased percentage than G-CSF | ||
| DC T Cells | DC2 Preferentially | DC2 Preferentially | |
| DC2 Preferentially | 20%, range 10-30% | ||
| B Cells | 10%, range 5-15% | ||
| NK Cells | 7.5%, range 5-10% | Increased percentage than G-CSF | |
| Granulocytes | 5.2%, range 0.6-10% | ||
| CD34+ Stem Cells | 0.3%, range 0.1-0.5% | Higher CD34+CD38- | Higher CD34+CD38- |
Mobilized leukopaks, which provide a rich source of hematopoietic stem cells compared to traditional leukopaks, are collected through the same process of leukapheresis, but the donor is prepped for the procedure with an injection of G-CSF (Granulocyte-Colony Stimulating Factor, also known as Neupogen®), and/or Mozobil. These growth factors stimulate the bone marrow to produce a large number of hematopoietic and progenitor stem cells and mobilizes them into the peripheral blood stream, leading to a larger collection of immune cells. The use of G-CSF arose as an alternative for bone marrow transplants, as it decreases pain and the risk of complications for the donor. Based on the mobilization schedule and drug used, different cell populations can be recovered as reviewed in table 2 above1.
At Caltag Medsystems, we offer products for all your cell isolation and immunology research needs, including leukopak units from healthy, normal human donors as well as mobilized leukopaks. In addition, we work with suppliers who can isolate PBMCs and more defined cell subsets through negative/positive magnetic bead isolation and provide these products in customized aliquots. Suppliers donor centres also have access to recallable donors with known HLA-types.
We support the ability to customize samples based on requirements or protocol needs and ensure your sample is collected from consented donors under IRB-approved protocols in use at our suppliers donor centers. Donor demographic information is available, including age, gender, smoking status, ethnicity, medication and surgical history. For more information or to speak with a specialist, please contact us at techsupport@caltagmedsystems.co.uk.